
7 Reasons to Choose Central and Eastern Europe as Your Go-To Destination for Clinical Trials
The global market for CROs is booming, projected to grow from $52.19 billion in 2023 to approximately $68.92 billion by 2027 (link to the source). Despite this, many clinical trials face significant delays, with 80% running over a month behind schedule, leading to substantial financial losses. These challenges often require rapid, effective strategies to rescue the trials.
Central and Eastern European countries (CEE) stand out as ideal for such interventions due to their fast enrollment capabilities and streamlined regulatory processes. With a large pool of treatment-naïve subjects available due to specific national healthcare systems, these countries offer a robust environment for getting clinical trials back on track efficiently.
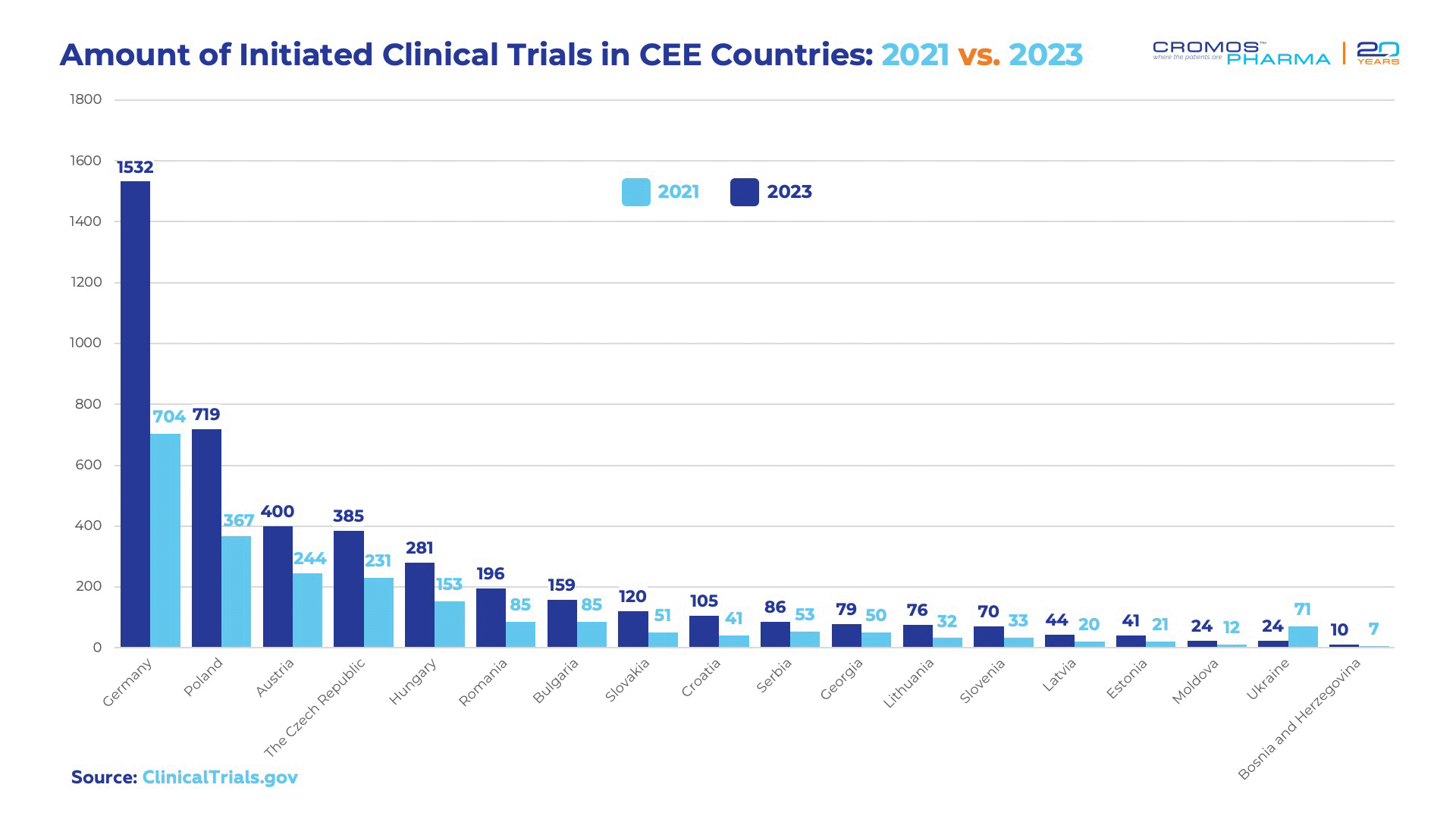
The appeal of the region in clinical research is also reflected in the growing number of trials being conducted in the region. For instance, data from the ClininicalTrials.Gov showed a significant increase in the number of studies, highlighting a trend where more sponsors are choosing CEE for their clinical trials. In 2021, the total number of initiated trials in the region was 2,260, and in 2023 this number had nearly doubled to 4,351. This growth underscores the region’s rising prominence and attractiveness for clinical research.
This article will explore 7 reasons why this region is becoming a favored destination for clinical research, demonstrating its growing importance in the global CRO market.
1. CEE’s Crucial Role in FDA Approvals
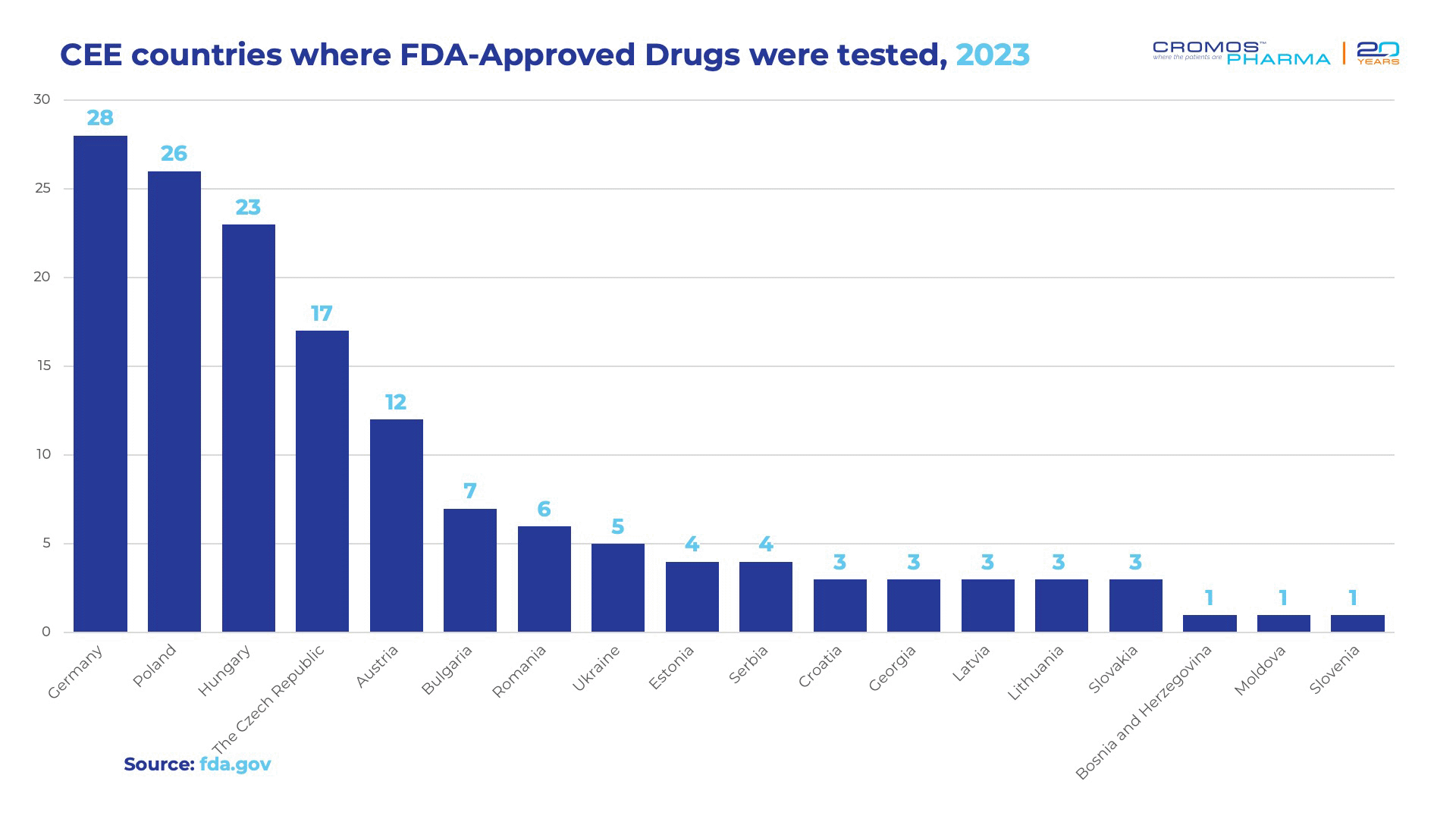
In 2022, the FDA’s Center for Drug Evaluation and Research approved 37 new drugs, with 29 of them undergoing clinical trials in Central and Eastern Europe. This trend continued into 2023, where 41 out of 55 FDA-approved drugs —equating to 75%—were tested in CEE countries (either solely or among other global geographies). This data highlights the significant role that CEE plays in global clinical trials, emphasizing the region’s importance for pharmaceutical companies seeking FDA approvals.
Below is a table detailing the CEE countries where these FDA-approved drugs were tested. It’s important to note that a single drug might have been tested in multiple CEE countries, further underlining the region’s pivotal role in the clinical trial process.
2. Proven Clinical Quality Standards
The FDA is responsible for inspecting clinical trial sites, research organizations, and facilities to ensure they comply with relevant regulations and standards for safety, quality, and efficacy in clinical research. FDA inspections are crucial for maintaining quality and safety from the outset of any clinical trial. As the number of international clinical studies in CEE rises, so does the frequency of FDA inspections in the region.
According to the data presented, CEE countries have achieved some of the highest rates of No Action Indicated (NAI) outcomes in 2024, highlighting their excellence in clinical research operations. Impressively, 75% of all inspections in the region received an NAI outcome, demonstrating strong compliance with regulatory standards. In comparison, this figure is around 65% in the US and Western Europe, highlighting the high quality of clinical research in CEE.
3. Large Pool of Patients
CEE offers a significant advantage for clinical trials due to its large and diverse population, which exceeds 250 million people. The region’s population is also genetically diverse, which is beneficial for trials requiring a wide range of participants, enhancing the generalizability of the results.
In addition, many CEE countries have healthcare systems that focus on large urban hospitals, making patient recruitment more efficient. Germany, with its population of over 83 million, plays a significant role in this capacity, offering a vast and diverse patient base for clinical research. Countries like Poland, Romania, and Hungary, each with populations between 10 to 40 million, contribute substantially to this pool, making CEE a strategic choice for pharmaceutical companies aiming for broad and inclusive clinical trials.
4. Streamlined and Harmonized Regulatory Processes
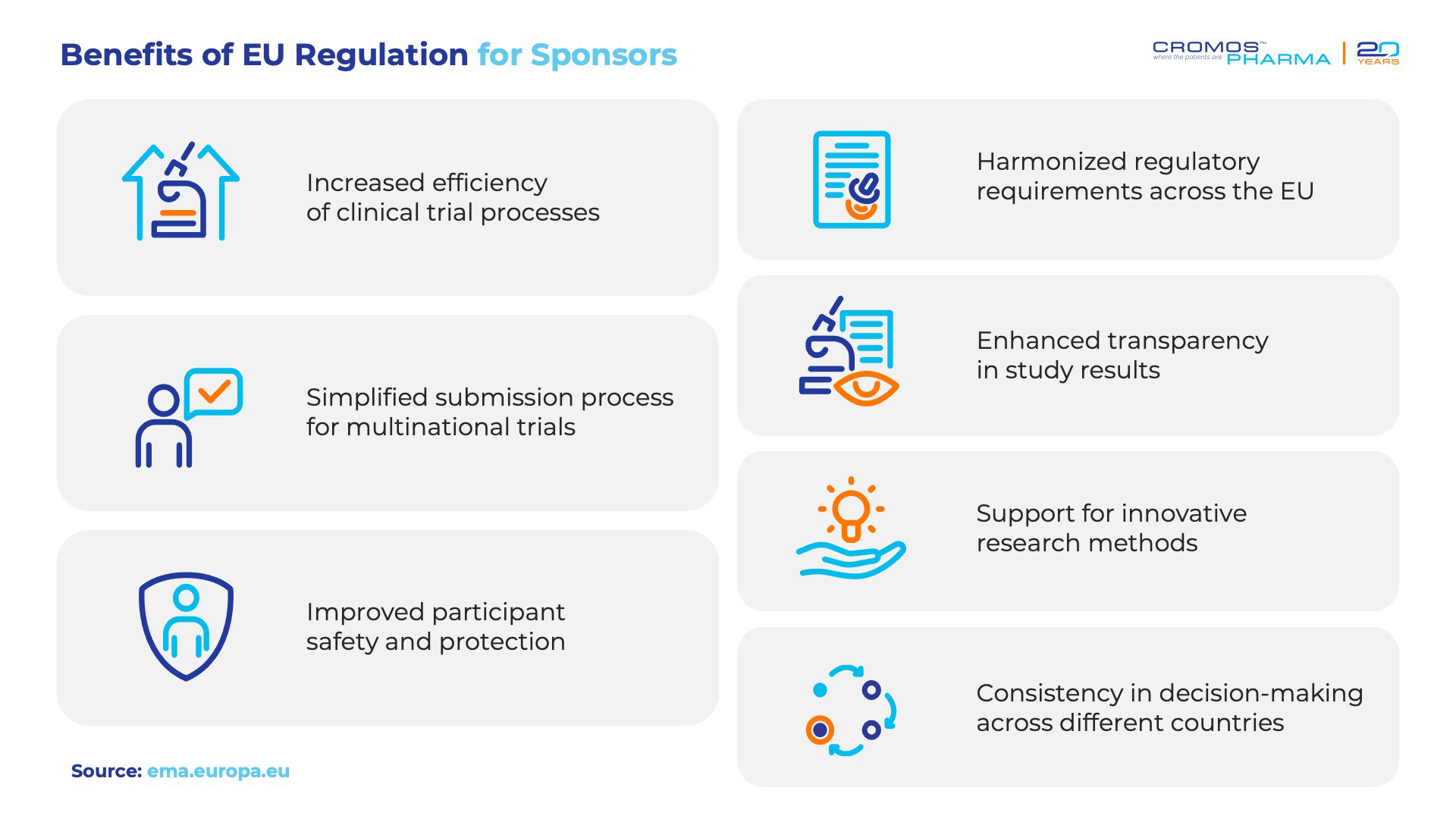
The regulatory environment in Central and Eastern Europe (CEE) is increasingly aligned with European Union (EU) standards, offering significant advantages for clinical trial sponsors. This alignment is largely driven by the EU Clinical Trials Regulation (EU No 536/2014) and the implementation of the Clinical Trials Information System (CTIS). These developments have simplified the process of submitting and managing clinical trials across EU countries, enhancing efficiency and reducing administrative burdens.
Since its introduction on January 31, 2022, CTIS has revolutionized the way multinational trials are conducted. Sponsors can now submit a single application for trials spanning multiple countries, streamlining the entire process. This system harmonizes regulatory procedures, ensuring that clinical trials conducted in CEE countries, as part of the EU, benefit from standardized regulations that promote faster approval timelines.
Under this system, clinical trial applications must be submitted through CTIS, where Parts I and II of the documentation are reviewed in parallel. This simultaneous review process typically takes about 6.5 weeks, leading to a total review and approval time of up to 8.5 weeks, with no Requests for Information raised during the review process. The result is a more predictable and efficient regulatory environment that encourages the initiation of clinical trials across multiple EU and EEA countries.
Beyond the EU, non-EU countries such as Georgia, Ukraine, and Moldova have also developed robust regulatory frameworks that align closely with international standards. These countries typically achieve approval timelines of up to 10 weeks, reflecting their commitment to maintaining high regulatory standards while facilitating the conduct of clinical trials.
However, in Serbia, the approval process may take longer, extending to approximately up to 15 weeks due to specific local regulatory requirements. Despite this, the overall trend in the region is toward greater harmonization and efficiency, making Serbia an attractive destination for clinical trial sponsors.
In conclusion, the streamlined and harmonized regulatory processes in CEE, supported by EU regulations and CTIS, create a conducive environment for clinical trials. Sponsors benefit from faster approvals, reduced administrative complexity, and the ability to conduct multinational trials with greater ease, making the region a pivotal player in global clinical research.
5. Recruitment Rates
CEE stands out for its rapid patient recruitment in clinical trials, often running 2-4 months ahead of schedule compared to the US and Western Europe. This advantage makes the region highly attractive to companies seeking to meet their recruitment goals efficiently. The region’s success is driven by several factors, including a large pool of treatment-naive patients, limited access to preventive medicine, and fewer competing trials.
6. Competitive Cost Advantage
The cost of conducting clinical trials in СEE is significantly lower than in the United States and Western Europe. On average, clinical trials conducted in CEE are 30% to 50% more cost-effective. This cost efficiency is due to lower operational expenses, such as site fees, investigator grants, and patient recruitment costs, without compromising the quality of data or compliance with regulatory standards.
7. Highly skilled and experienced clinical personnel
High-quality clinical research in CEE is driven by highly skilled and motivated investigators, whose expertise rivals that of their counterparts in the US and Western Europe. The region’s strict adherence to ICH-GCP standards, closely monitored by local authorities, ensures that all clinical trials meet rigorous global standards. The relatively lower density of trials in CEE allows investigators to dedicate more time and attention to each study, enhancing the quality of data collected and patient care. This environment also enables the delivery of innovative therapies to patients with limited treatment options.
Moreover, the centralized healthcare systems in Eastern Europe channel patients into specialized centers, often large university hospitals, where clinical trials are concentrated. This setup not only ensures that investigators are highly experienced in conducting specialized research but also facilitates the efficient recruitment of patients who meet study criteria, leading to faster and more reliable enrollment. The combination of skilled personnel, rigorous standards, and an optimal research environment makes CEE an ideal location for conducting high-quality clinical trials.
Cromos Pharma: Pioneering Clinical Research in Central and Eastern Europe
Cromos Pharma has been a pivotal player in clinical research across Central and Eastern Europe since extending its operations to the region. Established in 2004, our offices across key CEE nations have built a robust track record of significant achievements for global sponsors, supported by profound expertise and steadfast commitment.
Cromos Pharma manages a wide range of clinical trials in CEE, including innovative medicines, generics, and biosimilars. Our experienced teams provide tailored services to international pharmaceutical and biotech companies, leveraging high recruitment rates in the region to deliver superior data quality. This extensive experience across diverse markets enables Cromos Pharma to efficiently meet or exceed recruitment timelines consistently.
For inquiries or to explore how Cromos Pharma can amplify your next clinical project in CEE, please feel free to contact us at inquiry@cromospharma.com. We are here to enhance your research endeavors with meticulous precision and dedication.






























