
Clinical Trials in Romania – So Much to be Discovered
With a growing population of 19,4 million inhabitants, Romania is the twelfth-largest country in Europe and the seventh-most populous member state of the European Union. With its clinical research activity on the rise, it is currently the home to more than 500 clinical trials.
In recent years, Romania has become a popular destination for international pharmaceutical companies seeking to conduct clinical trials in Europe. Romania has a well-developed healthcare system, well-trained investigators, a favorable regulatory environment, and a diverse patient population that is eager to participate in clinical trials.
Country Overview
Romania is a sovereign state, located at the crossroads of southeastern and central Europe and situated in the northern part of the historically significant Balkans, bordering the Black Sea. Its capital, Bucharest, is the 8th largest city in the European Union.
Additionally, Romania is one of the advanced European telemedicine hubs, where technology is considered a primary growth driver. The national telemedicine network includes two command centers at Floreasca Hospital in Bucharest and Clinical Emergency Hospital of Târgu Mureș, which cover 56 hospitals in 19 counties.
The Center for Innovation in Medicine is an organization based in Bucharest, focused on the innovation in healthcare. It is a non-governmental organization that aims to shorten the time between the emergence of innovations in medical sector and their application for patients benefit in Romania and elsewhere. It also serves as an independent platform for informed dialogue between all stakeholders in the field of precision medicine, digital health, immune-oncology, cancer research, biotechnology, systems biology, and other emerging scientific fields.
Demographics
The current population of Romania is 19,4 million. The country has a diverse population, represented by several ethnic groups, including Romanians, Hungarians, Bulgarians, Germans, Ruthenians and Ukrainians, Serbs, Croats, Jews, Greeks, Armenians, Turks, and Romani people. The population of Romania is relatively young, with a median age of 40 years.
Urbanization has been a significant demographic trend in Romania, with approximately 57% of the population living in urban areas.
Healthcare Sector
The healthcare sector in Romania has undergone significant changes in recent years, with a focus on improving the quality of care and expanding access to medical services. The following are some of the key aspects:
- Healthcare system: Romania has a public healthcare system that provides medical services to citizens through a network of state-run hospitals and clinics. The government also offers a basic health insurance package to citizens, which covers the cost of medical services, medicines, and medical procedures.
- Private healthcare sector: In addition to the public healthcare system, there is a growing private healthcare sector in Romania, which offers high-quality medical services to patients. The private healthcare sector includes both for-profit and non-profit hospitals, clinics, and medical centers.
- Healthcare infrastructure: Romania has a well-developed healthcare infrastructure, with a network of hospitals and clinics that provide medical services to patients.
- Healthcare workforce: Romania has a large and qualified healthcare workforce, including doctors, nurses, and other healthcare professionals. However, there are still some shortages of medical personnel in some areas of the country, particularly in rural areas.
- Healthcare financing: The healthcare sector in Romania is funded by the government, through the public healthcare system, and by private insurance companies. The government is also working to increase investment in the healthcare sector, to improve the quality of care and to expand access to medical services.
The current life expectancy in Romania is 76.5 years. The share of the population aged 65 increased by almost half a million people, from 16.1 percent in 2011 to 19 percent in 2021. The country provides 6.9 hospital beds per 1,000 inhabitants.
Reasons to Conduct Clinical Trials in Romania
Over the past two decades, Romania has developed a thriving healthcare sector with high patient availability and fast recruitment timelines. Thus, it has become an attractive destination for clinical research. Apart from that, Romania has become more popular in the recent years due to:
- Access to a large pool of patients: Romania has a large patient population, making it easier to recruit participants for clinical trials.
- Lower cost: Romania has lower labor and operational costs compared to other countries in Europe, which translates into lower costs of clinical trial conduct.
- Experienced medical staff: Romania has a highly skilled medical workforce, with many doctors and nurses who are experienced in conducting clinical trials.
- Efficient regulatory environment: The Romanian regulatory environment is efficient and supportive of clinical trials, with a streamlined process for obtaining approvals and completing trials.
- Healthcare infrastructure: Romania has a strong healthcare infrastructure, with well-equipped medical facilities and easy access to medical supplies and equipment.
There are many high-quality investigational sites in Romania with abundant teaching hospitals and university clinics. There are 17 medical universities in Romania, while the number of university clinics stands at 39, which diagnostic capabilities are comparable to the highest international standards. In terms of Romania’s innovation centers, the city of Iasi, which is also the second-largest city in terms of the number of universities, is quite significant. The key medical imaging cluster in Romania and the whole EU is in Iasi. The cluster helps innovators and connects private healthcare organizations with businesses in the IT and software sectors. Iași’s Tehnopolis Technological Park also offers biotechnology lab services.
Starting in 2022, The Ministry of Research, Innovation and Digitization in partnership with the POLITEHNICA University of Bucharest began organizing annual “Romania of the Future” forums, which include exhibitions featuring the results of research and innovations, as well as panel sessions and discussions in the area of research and entrepreneurship.
Romania: Quick Facts
Regulatory approval process
Romania recently implemented the Clinical Trials Information System (CTIS). This puts the deadline for a reply after submission at 60 days.
Agreements with sites and investigators
Contract with the hospital/site and contracts with PIs are required. Both contracts, with the medical center and PI must be submitted to the EC.
Trial sites’ location
Main medical centers are located in University cities such as Bucharest, Cluj-Napoca, Iasi, Timișoara, Târgu Mureș, Oradea, Craiova and Constanța.
Legal entity
It is not mandatory to have a local CRO representative as the applicant in Romania. However, the entity conducting the clinical trial should be able to perform payments in national currency (RON) and to provide specific documents in Romanian language.
QP Declaration / GMP certificate
GMP certificate or/and Declaration of the QP that manufacturing site works in compliance with EU GMP are required for submission.
Documents requiring special attention
Informed Consent Form (ICF), investigational label and summary of the product characteristics in Romanian language should be customized with country specific information.
The study Synopsis must be submitted in Romanian language.
Official language
Essential documentation should be submitted in English. Patient-related documents, labels and the protocol synopsis must be translated into Romanian language.
According to OUG no 46/9th June 2021, when conducting clinical trials of medical devices, the information submitted by the manufacturer together with the medical device, provided in annex I of the 745/2017 of the Medical Devices Regulation, must be presented in Romanian language, along with the relevant official languages of the European Union. Information regarding patient documentation such as informed consent, patient recruiting materials, patient questionnaires, conformity assessments, safety notifications are also to be submitted in Romanian language.
Patient insurance
Local insurance policy accompanied by the general terms to the insurance and insurance certificates are needed for submission (with signed and dated copy stamped “corresponding to the original” issued by the insurance company).
Useful information
It is possible to submit the draft contract with obligation to provide the scan of a fully executed document within 45 days. However, a complete set documentation of at least one of participating sites should be included.
Snapshot of Romania’s Clinical Trials
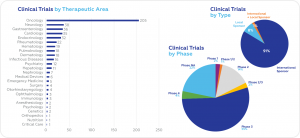
According to data obtained from clinicaltrials.gov, the largest share of ongoing clinical trials is in the field of oncology (205), followed by neurology (38), gastroenterology (36), and cardiology (35). Most ongoing trials are Phase 3 (55%). International Sponsors are responsible for 91% of ongoing trials.
Regulatory environment for Clinical Trials in Romania
Romania does not have a clinical trial registry of its own. It is an EU/EEA member, and it follows EU Clinical Trials Regulation no 536/2014. Protocol registration is done via Clinical Trials Information System (CTIS). Amendments to the submissions that predate February of 2023, for CTAs that were submitted under the old legislation (Clinical Trial Directive 2001/20/EC), utilizing EudraCT. Such clinical trials can continue, assuming that they will be completed by January 30, 2025. Processes will remain unchanged, and sponsors will therefore be able to submit substantial amendments and end of trial notifications as needed under the Directive. EudraCT will remain operational throughout the transition period to enable these trials to continue.
In order to perform clinical trials in Romania, it is required to obtain authorization from the National Bioethics Commission for Medicines and Medical Devices (CNBMDM) and the National Agency for Medicines and Medical Devices from Romania (ANMDMR). The ethical analysis carried out by the CNBMDM includes aspects covered by part I of the evaluation report for the authorization of an interventional clinical trial, as provided for in art. 6 of the Clinical Trials Regulation, and aspects covered by part II of the evaluation report are provided in art. 7 of the Clinical Trials Regulation.
According to the applicable regulations, the clinical trial protocol should provide:
- General information regarding the sponsor, investigators, and laboratories;
- Certain information regarding the investigated product, its use, and the target population;
- The clinical trial’s objectives;
- The selection and withdrawal of the subjects;
- The treatment administered to the subjects;
- The evaluation of the trial’s efficacy and safety;
- Statistical information;
- Quality assurance and quality control provisions;
- Ethical aspects;
- Data processing and data protection aspects;
- Financing, insurance, and certain other aspects are provided by the clinical trial guidelines.
CNBMDM and ANMDM are responsible for implementing laws and guidelines associated with medicinal products and medical devices. They are also responsible for the efficacy and safety of all medicinal products, for oversight of controlled drugs and substances, and for approving imported medical devices.
Cromos Pharma – your partner in International Clinical Research
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest period possible. We recruit highly educated and experienced staff that assures that each trial managed by our team in Romania produces exceptional data quality and reliable results.
Cromos Pharma combines global expertise with in-depth local experience and knowledge that translates into exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of the conducted trials.
If you are considering conducting clinical trials in Romania or in any other countries where Cromos Pharma is present, our team would be happy to answer any of your questions.






























