
Clinical Trials in Bulgaria – Country Profile for 2023
Bulgaria, located in Southeastern Europe, boasts a mature healthcare system and a growing pharmaceutical industry. Based on the significant number of clinical trials conducted per capita, Bulgaria is recognized as one of Europe’s formidable clinical research hubs. With around 12,000 patients enrolled annually, Bulgaria is an attractive proposition for pharmaceutical companies and CROs. There are currently 550 clinical trials being conducted in the country, acting as a further incentive to the Bulgarian Association of Clinical Research (BACR) to advocate for high standards in meeting all international requirements.
Some of the many advantages of conducting clinical trials in Bulgaria include the efficient recruitment of patients, competent medical staff, high quality medical facilities and a favorable regulatory environment. The costs of conducting trials are very competitive.
Country Overview
Bulgaria is bordered by Romania to the north, Serbia and North Macedonia to the west, Greece and Turkey to the south, and the Black Sea to the east. The capital, and largest city, is Sofia, followed by Plovdiv and Varna.
Over the past three decades, Bulgaria has transitioned from a highly centralized, planned economy into an open, market-based system that is now firmly part of the European Union. After a decade plagued by weak economic growth, high debt levels and loss of savings, Bulgaria has become a true and robust market economy. Their currency is the Bulgarian Lev.
Demographics
Bulgaria’s current population is 6.6 million, and the official language is Bulgarian. The three largest ethnic groups comprise of Bulgarians, (84.8%), Turks (8.8%) and Romi, (4,9%). The average life expectancy of 75.4 years. The process of urbanization in Bulgaria has resulted in 75.6 % of the population now residing in urban areas.
Healthcare Sector
The Ministry of Health is in charge of the country’s healthcare system, which is highly centralized, and the Bulgarian government has gone to great lengths to raise the standard of healthcare. To that end, there is an ongoing reform to modernize and increase the number of medical facilities.
In 2021 the Bulgarian pharmaceutical market grew by 8.5 percent. Many global pharmaceutical companies such as AbbVie, Amgen, Eli Lilly, Janssen, Merck Sharp & Dohme Corp. (MSD), Pfizer, AstraZeneca, Novartis, Roche, Sanofi in this market.
Bulgaria is viewed by many Contract Research Organizations (CRO’s) as a promising venue, so there is increasing competition between the US, French, German, and Swiss contingents. The US currently have a market share of 50%. With over 135 members, the active Bulgarian Association of Clinical Research (BACR) promotes high standards in clinical trial execution and adherence to international norms.
Reasons to Conduct Clinical Trials in Bulgaria
The country has a well-established healthcare system, a sizeable patient population, and a cost-effective environment in which to conduct clinical research. Bulgaria is also a popular destination among contract research organizations due to its:
- Centralized healthcare system and large specialized medical centers
- Stable and predictable regulatory environment enhanced by the harmonization of national legislation with the EU acquis
- Efficient process of patient recruitment
- Competent medical staff
- High quality of collected data
- Well-equipped investigational sites
Bulgaria is a home to many high-quality investigational sites in that stem from the strong presence of teaching hospitals, university clinics and postgraduate medical schools. There are 5 medical schools and 15 university clinics with clinical and research facilities and diagnostic capabilities equal to those found in Western countries. The regulatory authorities are strict in ensuring that GCP guidelines are implemented, and all investigators are trained in GCP.
Bulgaria: Quick Facts
Regulatory approval process
Bulgaria recently implemented the centralized EMA’s Clinical Trials Information System (CTIS), which reduces the review time to 60 days.
Agreements with sites and investigators
Contracts are required with all sponsors, PI’s and the institutions where the CT will be conducted.
Trial sites’ location
The main medical centers are in the major cities such as Sofia, Plovdiv, Varna, Burgas.
Legal entity
While it is not mandatory to have a local CRO representative as the applicant in Bulgaria, the entity conducting the clinical trial should be able to make payments in the national currency (BGN) and to provide specific documents in Bulgarian.
QP Declaration / GMP certificate
A declaration by the QP that the manufacturing site is compliant with the EU is required for submission.
Documents requiring special attention
The Informed Consent Form (ICF), written in Bulgarian should be customized with country-specific information.
Official language
Essential documentation should always be submitted in English, however, patient-related documents, labels and the Protocol synopsis must be translated into Bulgarian.
Patient insurance
A local insurance policy together with the general insurance terms and insurance certificates are needed for submission.
Useful tips
If more information is required during the assessment of documentation, The CA/EC can request an extension not exceeding a calendar month. The sponsor is required to reply within 12 calendar days, and failure to respond timeously will lead to the withdrawal of the submission.
Snapshot of Bulgaria’s Clinical Trials
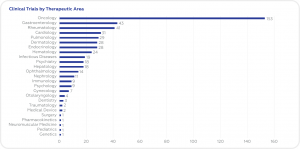
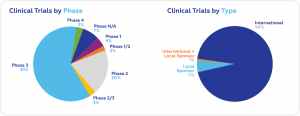
According to recent data obtained from clinicaltrials.gov, the largest share of ongoing clinical trials is in the field of oncology (153), followed by gastroenterology (43), rheumatology (41), and cardiology (31). Most ongoing trials are in Phase 3 (336). International Sponsors are responsible for 94% of ongoing trials.
Regulatory environment for Clinical Trials in Bulgaria
Effective from 31 January 2022, the EU Clinical Trials Regulation No 536/2014 (CTR) became applicable to all EU/ European Economic Area (EEA) Member States, thereby replacing the EU Clinical Trials Directive (2001/20/EC) (CTD). Starting from 31 January 2023, all new CTAs must be submitted under the EU Clinical Trials Regulation via the CTIS.
Amendments to the submissions made before 31 January 2023 under the old legislation (Clinical Trial Directive 2001/20/EC) may continue if the trials are anticipated to be completed by January 30, 2025. Processes will remain unchanged, and sponsors will therefore be able to submit substantial amendments and end of trial notifications as needed under the Directive.
To perform clinical trials in Bulgaria according to the CTR, CTIS User Administrators/Users are required to submit the application dossier a harmonized format and consisting of:
- Part I: Scientific Review documents and
- Part II: Ethical Review documents.
A comprehensive list of documents is required as part of Clinical Trials Regulation No 536/2014, Annex I. The CTIS User Administrator/User can either submit Part I and Part II together or alternatively submit Part I first, followed by Part 2.
Cromos Pharma – your partner in International Clinical Research
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest period possible. We recruit highly educated and experienced staff that assures that each trial managed by our team in Bulgaria produces exceptional data quality and reliable results.
Cromos Pharma combines global expertise with in-depth local experience and knowledge that translates into exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of the conducted trials.
If you are considering conducting clinical trials in Bulgaria or in any of the other countries where Cromos Pharma is present, our team will be happy to answer any of your questions.






























