World Hemophilia Day 2023
Every year April 17 is observed as World Hemophilia Day. It brings attention to a rare bleeding illness that impairs the blood’s ability to clot and offers an opportunity to spread awareness of the illness, its signs and symptoms, available treatments, and the difficulties that people with Hemophilia and other bleeding disorders encounter every day.
World Hemophilia Day was established in 1989 by the World Federation of Hemophilia (WFH). Each year, the WFH chooses a theme to highlight a specific aspect of Hemophilia and other bleeding disorders. This year’s theme is “Access for All: Prevention of bleeds as the global standard of care”. The WFH and its partners around the world organize a wide range of events and activities, including educational seminars, fundraising campaigns, blood drives, and advocacy initiatives to improve access to care and treatment options for those affected by Hemophilia.
Hemophilia is a genetic bleeding disorder that causes the blood to clot improperly. This may result in both spontaneous bleeding and bleeding after injury or surgery. Blood contains a variety of clotting proteins (factors) that can aid in stopping of the bleeding. Low amounts of either factor VIII or factor IX are seen in patients with Hemophilia.
There are 3 types of Hemophilia:
- Hemophilia A is caused by a deficiency in clotting factor VIII. It is the most common type of Hemophilia and affects around 1 in 5,000-10,000 males.
- Hemophilia B is caused by a deficiency in clotting factor IX. It is less common than Hemophilia A, affecting around 1 in 25,000-30,000 males.
- Hemophilia C is caused by a deficiency in clotting factor XI, it is a rare disease. People with Hemophilia C do not experience severe bleeding symptoms.
The current treatment of Hemophilia consists of replacing the missing blood clotting factor. Clotting factor concentrates, which are used as therapeutic products, are usually delivered via intravenous injection. Clinicians typically prescribe treatment products for episodic or prophylactic care. Episodic care is used to stop bleeding episodes; prophylactic care is used to prevent bleeding episodes from occurring.
Gene therapy is the vanguard in the treatment of Hemophilia. It aims to introduce functional copies of the deficient clotting factor genes into the patient’s cells, thereby restoring its production and reducing the frequency and severity of bleeding episodes. This can be achieved using a variety of techniques, such as viral vectors, which are modified viruses that can deliver therapeutic genes to the patient’s cells.
Several gene therapy products for hemophilia are being tested in clinical trials and have shown promising results, with some patients achieving long-lasting improvements in their clotting factor levels and a reduced need for regular infusions of clotting factor concentrates.
In November of 2022 the U.S. Food and Drug Administration approved Hemgenix (etranacogene dezaparvovec), first gene therapy to treat adults with Hemophilia B. It is the first adeno-associated virus vector-based gene therapy that is now available to patients.
Hemophilia Statistics
Over the last 20 years, the number of patients with Hemophilia in the world increased substantially from 70.000 to 256.840 people.
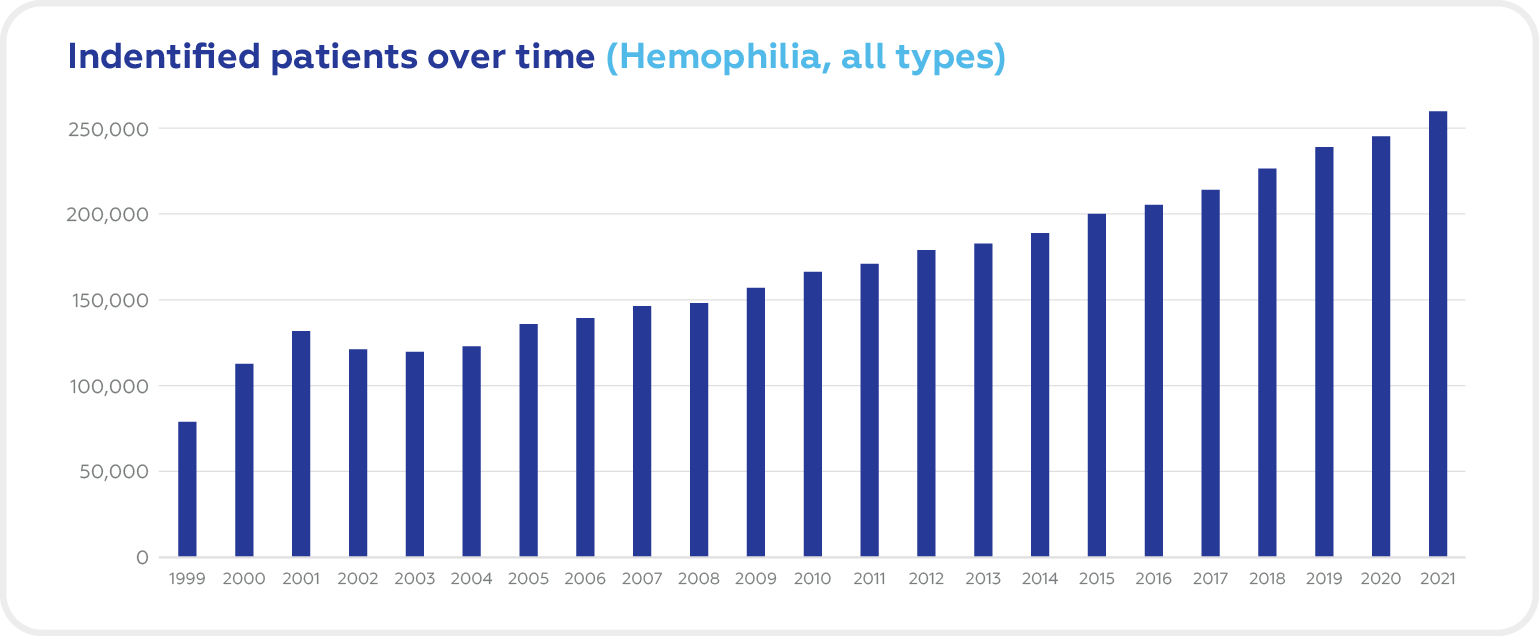
Source: World Federation of Hemophilia Report on the Annual Global Survey 2021
In the United States, Hemophilia A affects 1 in 5,000 male births. About 400 babies are born with Hemophilia A each year. Most people with Hemophilia are diagnosed at a very young age of 1 to 36 months. In 2/3 of cases, these patients have a family history of Hemophilia.
Hemophilia Clinical Trials
There are now 166 ongoing clinical trials in Hemophilia A and 66 studies in Hemophilia B according to Clinicaltrials.gov (recruiting, not yet recruiting, and active, non-recruiting). The top regions in the Hemophilia clinical trials of both types are North America, Europe, and East Asia.
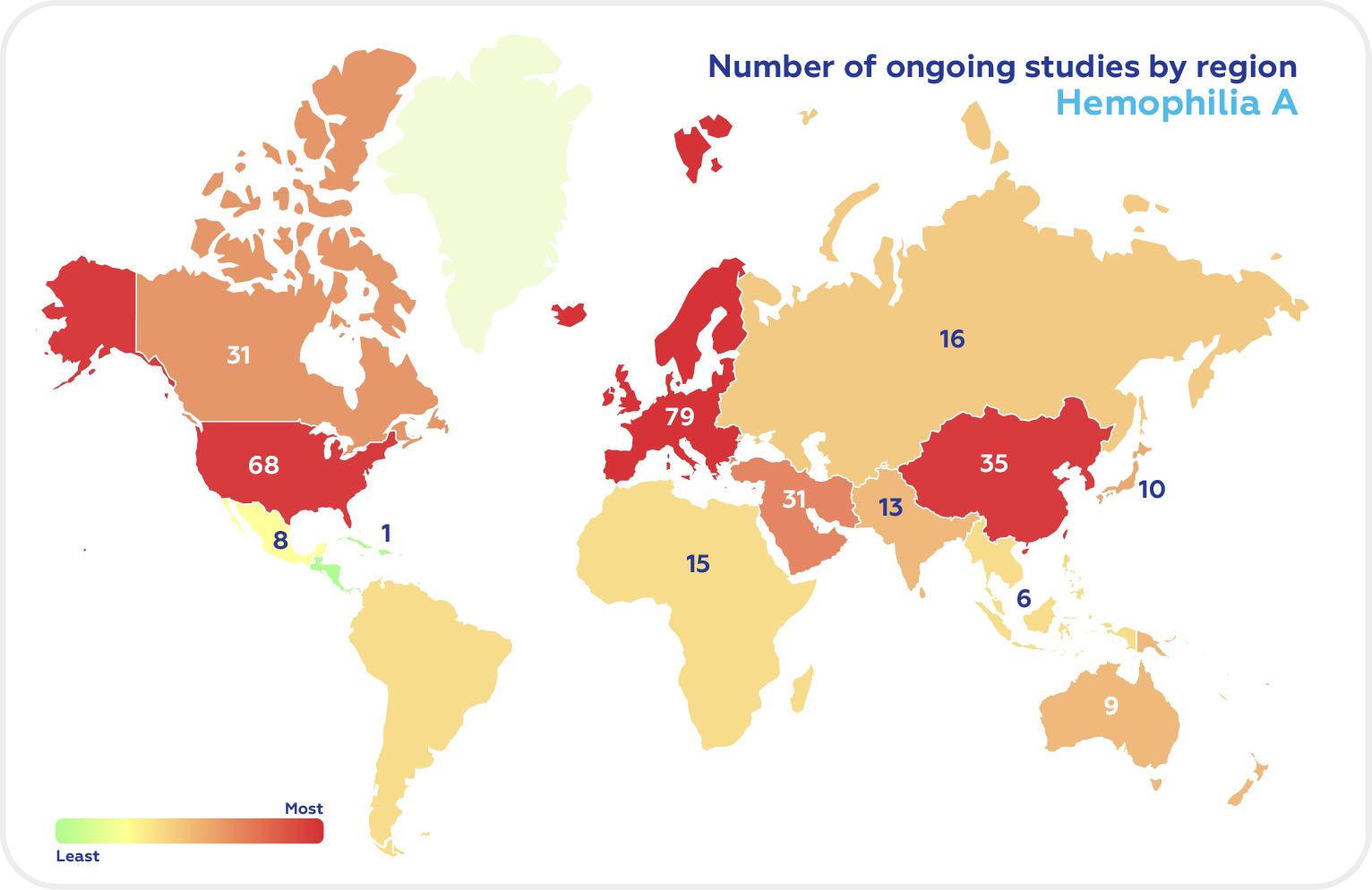
As of October 2022, the US has the highest number of Hemophilia A clinical trials, followed by the UK, Italy, France, Germany, Japan, Spain, Russia, Turkey, and Canada.
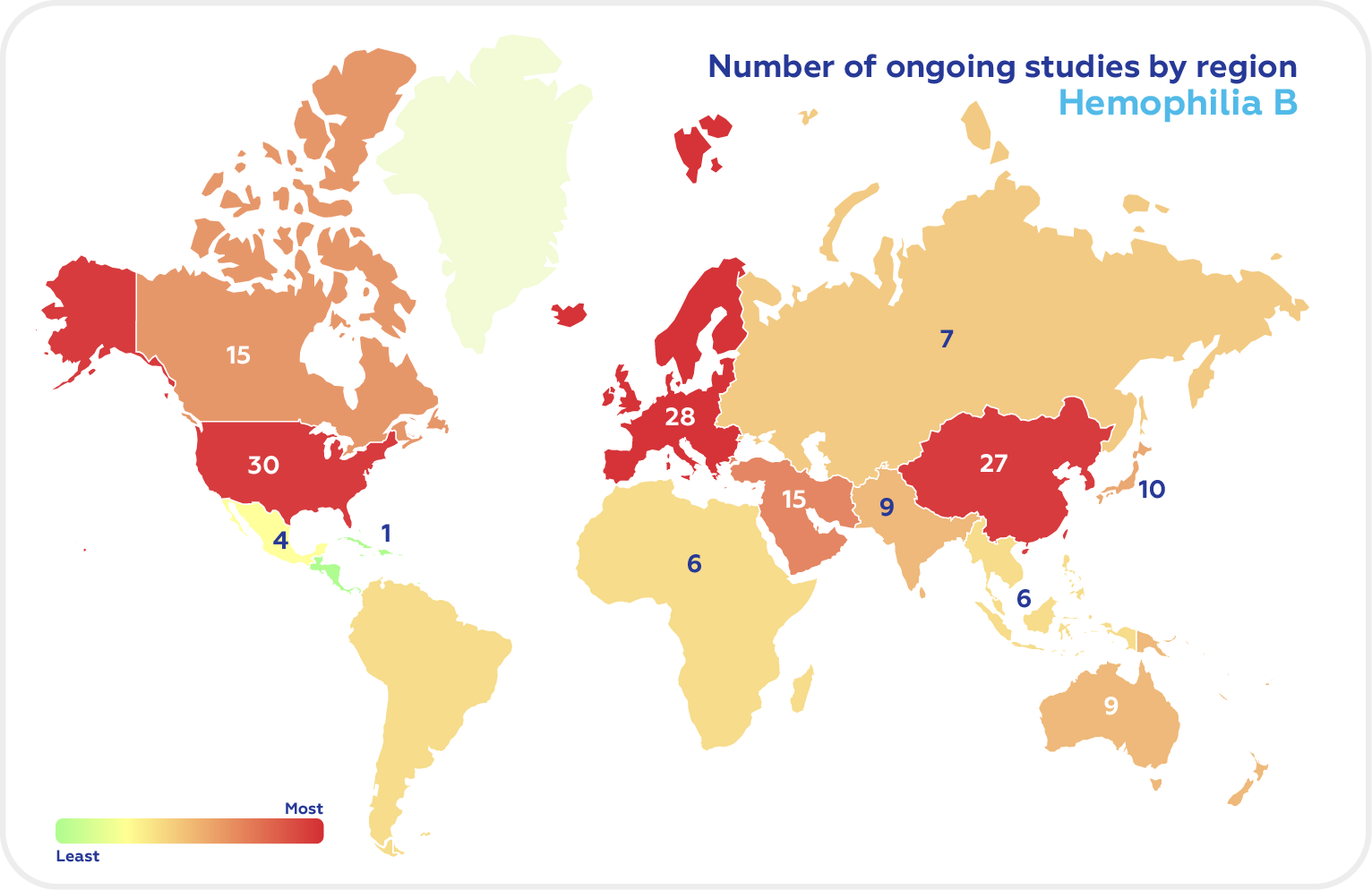
Among the G7 countries, the majority were conducted in the US with UK having the highest proportion of Hemophilia B clinical trials.
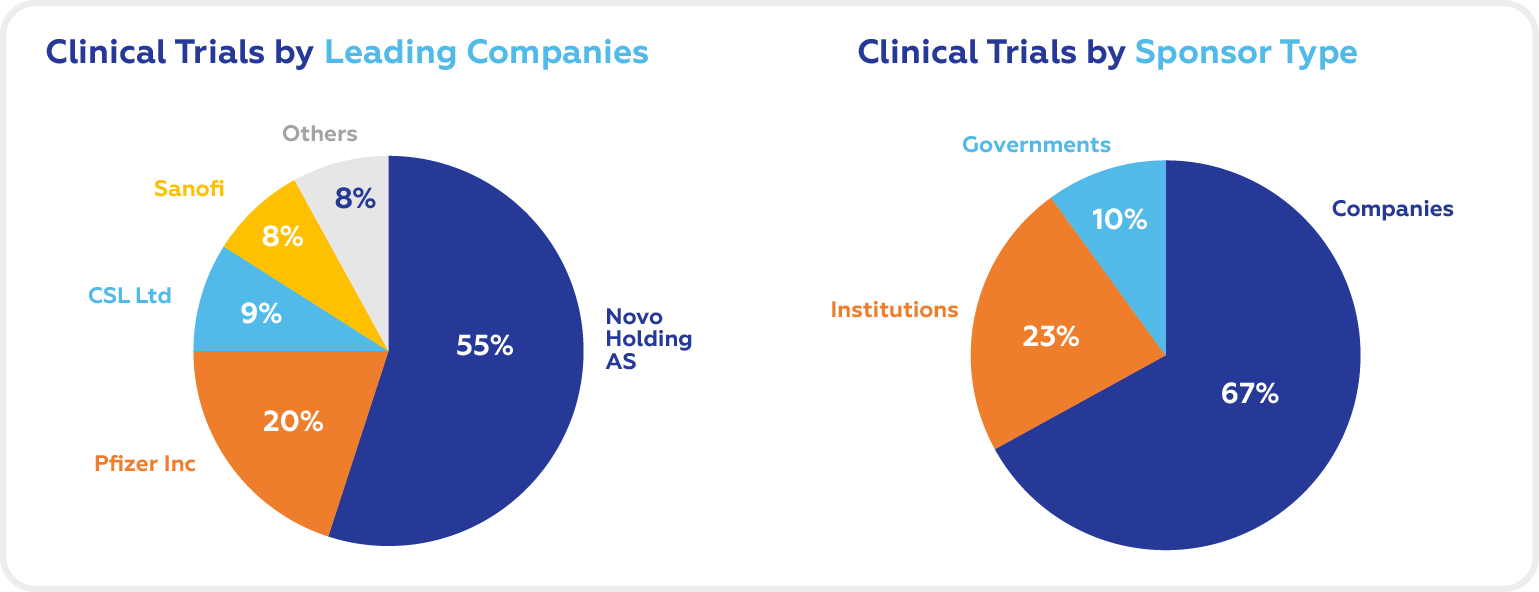
National and international foundations, companies, and governments are the three main sponsor types for clinical studies for Hemophilia B. The market is dominated by biotech and Pharma companies.
The leading companies in the Hemophilia B clinical trials space are Novo Holdings AS, Pfizer Inc, CSL Ltd, and Sanofi.
Cromos Pharma has extensive experience in managing all aspects of clinical trials in hematology, in all phases, including rescue, post-marketing and observational studies. Hematology trials represent a substantial part of the company’s research portfolio.






























