
Poland: A Thriving Hub for Biotech Advancements and Clinical Trials
Poland has emerged as a leading destination for biotech professionals seeking a dynamic and conducive environment for conducting clinical trials. With its robust healthcare infrastructure, highly skilled workforce, supportive regulatory framework, and favorable economic conditions, Poland offers a compelling landscape for biotech advancements. This article aims to delve into the key factors that have made Poland an attractive destination for clinical trials and highlight its growing influence in the biotech industry.
One of Poland’s major strengths lies in its diverse and sizable patient population, offering access to a broad range of potential study participants. The country’s population of over 38 million individuals represents a diverse genetic profile, allowing for comprehensive clinical research. Additionally, Poland’s centralized healthcare system ensures efficient patient recruitment and follow-up, thereby expediting trial timelines.
In addition to its scientific and logistical advantages, Poland offers significant cost savings compared to other European countries and the United States. The lower costs associated with conducting clinical trials in Poland stem from favorable exchange rates, competitive operational expenses, and lower patient recruitment expenses. These factors make Poland an attractive option for biotech companies seeking to optimize their research and development budgets without compromising on quality.
Poland actively fosters collaborations between academia, research institutions, and industry stakeholders. Numerous public-private partnerships and research clusters have been established to encourage knowledge sharing and interdisciplinary cooperation. Such initiatives facilitate the exchange of expertise, resources, and data, thereby driving innovation in clinical trial methodologies and accelerating the development of novel therapies.
Country Overview
Poland is a country located in Central Europe. It covers an area of approximately 312,000 square kilometers, making it the ninth-largest country in Europe. It shares its borders with Germany to the west, the Czech Republic and Slovakia to the south, Ukraine and Belarus to the east, and Lithuania and Russia (Kaliningrad Oblast) to the northeast. The capital and largest city of Poland is Warsaw. Polish is the official language. The country has a rich cultural heritage, it has made significant contributions to various fields, including literature, music, and science.
Poland has a mixed-market economy and is considered one of the most robust economies in Central Europe. Key sectors include manufacturing, information technology, automotive, pharmaceuticals, and agriculture. The country is a member of the European Union, the United Nations, and the World Trade Organization.
Demographics
Poland is the ninth-most populous country in Europe, with a population of approximately 38 million people. Poland has a relatively balanced age distribution. The median age in the country is around 40 years, indicating a moderate aging population. The largest age group is 15-64 years, it represents around 63%. Poland has a significant urban population, with most people living in urban areas. Cities such as Warsaw, Kraków, Łódź, and Wrocław are the major urban centers.
Most of the Poland’s population identifies as ethnically Polish, with Polish culture and language being predominant. However, Poland is also home to small ethnic minorities, including Ukrainians, Germans, Belarusians, and others.
Healthcare System
The healthcare in Poland is provided through a universal insurance, which means that it is available to all Polish citizens and legal residents. The funding is provided through a combination of public funds and contributions from employees and employers.
The National Health Fund (NFZ) is the central institution responsible for managing and financing the healthcare system. It oversees the allocation of funds, contracts with healthcare providers, and reimburses medical services.
Poland’s healthcare infrastructure is characterized by modern facilities, well-equipped research centers, and advanced medical technologies. The country’s network of specialized hospitals, clinics, and research institutions provides an ideal environment for conducting clinical trials across various therapeutic areas. Biotech professionals benefit from state-of-the-art laboratories, imaging facilities, and expert medical personnel, enabling them to execute trials with precision and accuracy.
Poland is renowned for its highly skilled workforce, including physicians, nurses, and clinical research professionals. Many Polish researchers have gained international recognition for their contributions to the biotech field. The country’s universities and research institutes produce a steady stream of talented graduates, ensuring a sustainable pool of professionals for clinical trials. Furthermore, Poland’s proficiency in multiple languages, including English, makes it easier for international biotech professionals to collaborate seamlessly.
Reasons to Conduct Clinical Trials in Poland
The advantages of selecting Poland as the destination for conducting clinical research include:
- Solid track record of generating high-quality clinical research data
- Large population of 38.15 million provides great potential for patient recruitment
- Abundance of treatment-naïve patients in a wide range of therapeutic areas
- Patients are eager to participate in clinical trials as a means of accessing novel therapies
- Highly skilled, qualified, experienced, and motivated investigators and site staff
- Large network of specialized medical facilities located around major urban centers
- Lower costs – on average 30% below the US – due in part to efficiencies in patient recruitment and moderate salaries and fees
- EU member state since 2004
Snapshot of Poland’s Clinical Trials
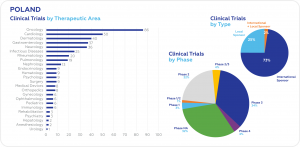
A total of 413 clinical trials were initiated between 1 June 2022 and 1 June 2023. According to data from clinicaltrials.gov, the largest number of clinical trials were conducted in oncology (86), followed by cardiology (50), dermatology (40), and gastroenterology (37). Most trials are in Phase 3 (34%). International sponsors conduct 73% of all ongoing clinical trials.
Poland: Quick Facts
Regulatory approval process
The application must be uploaded onto the Clinical Trials Information System (CTIS). Parts I and II of the documents are assessed in parallel within 45 calendar days (60 calendar days in total for review and approval).
Agreements with sites and investigators
Usually, 2 agreements should be signed – with an institution and with a PI. The draft contract must be submitted and if not available, statements regarding study funding and financial operations in the study must be submitted. Having a country-specific Clinical Trial Agreement template is helpful in the negotiation process.
EC review and approval
CEC is the only ethics body involved in the review process (Part II Ethical Review). Submission should be done via Clinical Trials Information System (CTIS).
Favorite trial sites’ locations
Main medical centers are located in major cities such as Warsaw, Kraków, Łódź, Wrocław, Poznań.
Legal entity
For non-EU Sponsors, an EU legal representative is required. CTIS application form contains sections that should be filled out in the Polish language. Submission to CEC needs to be done in either Polish or in both English and Polish. The assistance of local staff is therefore recommended.
QP Declaration / GMP certificate
Declaration from the QP stating that the manufacturing site operates in compliance with the EU GMP is required for submission.
Documents requiring special attention
Informed Consent Form (ICF) should be completed in the local language and should contain country-specific information.
Official language
Essential documentation should be submitted in English. Patient-related documents, Labels, protocol synopsis must be translated into the Polish language.
Patient insurance
Global insurance agreement with the local legal entity in the country is required. A further requirement is that a local contact who speaks the Polish language should be available to assist the patient with any questions.
Useful tips
Initial submissions made before 31 January 2023 and submitted under the old legislation (Clinical Trial Directive 2001/20/EC) can continue, based on the assumption that the trials will be completed by January 30, 2025. Processes will remain unchanged, and sponsors will therefore be able to submit substantial amendments and end-of-trial notifications as required under the Clinical Trial Directive.
Regulatory Environment for Clinical Trials in Poland
On 31 January 2022, the EU Clinical Trials Regulation No 536/2014 (CTR) became applicable in all EU/ European Economic Area (EEA) Member States, thereby replacing the EU Clinical Trials Directive (2001/20/EC) (CTD).
Effective January 31, 2023, all new CTAs must be submitted under the EU Clinical Trials Regulation via CTIS.
To perform clinical trials in Poland, the CTR requires that all CTIS User Administrator/User submit a harmonized format of the application dossier consisting of Part I (Scientific Review documents) and Part II (Ethical Review documents).
The Clinical Trials Regulation No 536/2014, Annex I, requires that a comprehensive list of documents is submitted. The CTIS User Administrator/User can submit Part I and Part II either simultaneously or sequentially.
The General timeline from study submission until initial approval is 60 days (validation – 10 days; assessment – 45 days; decision – 5 days). Parts I and II can be assessed in parallel within 45 calendar days and up to 76 calendar days if there are any requests for information.
Poland has firmly established itself as a preferred destination for conducting clinical trials, offering biotech professionals an array of advantages. With its favorable regulatory environment, diverse patient population, state-of-the-art infrastructure, skilled workforce, cost-effectiveness, and collaborative research initiatives, Poland provides an optimal setting for driving biotech advancements. As the country continues to evolve its clinical trial landscape, it is poised to contribute significantly to the global biotech industry’s progress. Biotech professionals are encouraged to explore the vast opportunities that Poland offers and engage in transformative research that can improve patient outcomes worldwide.
Cromos Pharma – your partner in International Clinical Research
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest period possible. We recruit highly educated and experienced staff that assures that each trial managed by our team in Poland produces exceptional data quality and reliable results.
Cromos Pharma combines global expertise with in-depth local experience and knowledge that translates into exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of the conducted trials.
If you are considering conducting clinical trials in Poland or in any of the other countries where Cromos Pharma is present, our team will be happy to answer your questions.






























