Exploring Portugal’s Clinical Trials Landscape: Innovations, Infrastructure, and Opportunities
Portugal stands as a strategic choice for conducting clinical trials, offering a unique blend of opportunities for the global healthcare and pharmaceutical industries. With a population of over 10 million, Portugal boasts a high-quality healthcare system integrated with advanced research facilities and a well-educated medical community.
Despite its smaller size, Portugal’s diverse demographic and genetic backgrounds make it an ideal setting for wide range of clinical trials. The country’s commitment to healthcare innovation is reflected in the growing interest from international biotech and pharmaceutical companies. In recent years, Portugal has seen a noticeable increase in the number of clinical trials, leveraging its EU membership to ensure regulatory compliance and access to European markets.
Over the last two decades, Portugal has made significant strides in establishing a solid infrastructure for clinical trials, which is now beginning to yield substantial results. The increase in multinational collaborations and the support from both public and private sectors are propelling Portugal to the forefront of clinical research in Europe.
This article explores the current landscape of clinical research in Portugal, shedding light on the factors that make it a compelling destination for future studies.
Country overview
Portugal is located at the southwestern tip of Europe, bordered by Spain and the Atlantic Ocean. Its strategic position enhances its connectivity with Europe and the Atlantic, fostering economic and maritime links worldwide.
Covering approximately 92,090 square kilometers, as noted by the United Nations, Portugal’s compact size facilitates logistics and research activities, making it an efficient hub for clinical trials.
Demographics
As of 2023, Portugal’s population stands at approximately 10.53 million, showcasing a rich cultural diversity with significant immigrant communities, with Portuguese as the dominant language.
The country enjoys robust health indicators with a life expectancy of 82 years and boasts a strong innovation profile, ranking 30th in the Global Innovation Index due to its vibrant industrial sectors and research prowess. The Human Capital Index score of 0.8 highlights the ongoing investments in health and education, underscoring Portugal’s dedication to development.
Socioeconomics
Lisbon, the national capital of Portugal, and Porto, its second most populous city, are central hubs for economic and research activities in the country. These cities host a large number of potential clinical research participants due to their significant urban populations and advanced healthcare facilities.
According to the latest UN data, Portugal’s major trade partners include Spain, France, and Germany. The country is deeply integrated into the European Union, enhancing its economic ties and facilitating trade and investment that are crucial for sectors like clinical research. Additionally, Portugal’s stable diplomatic relationships within the EU and globally support its access to essential resources for healthcare and scientific research.
At a Glance: Portugal’s Pharmaceutical Industry
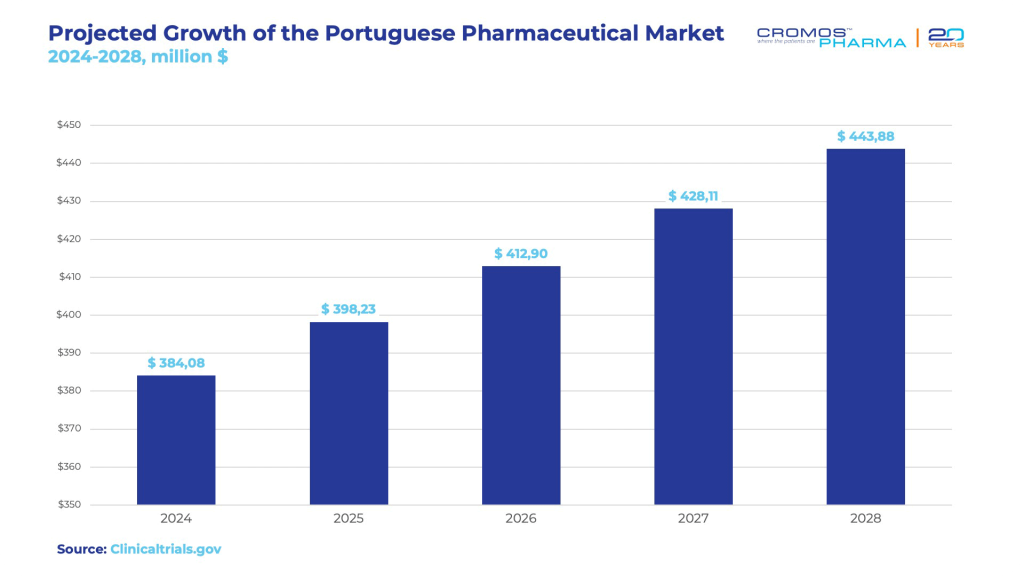
- By 2028, the Portuguese pharmaceutical market is expected to grow from around $370.44 million in 2023 to approximately $443.88 million, reflecting a compound annual growth rate (CAGR) of 2.9%.
- In 2023, Portugal was the 10th largest pharmaceutical producer in Europe, closely following the Netherlands.
- As of recent data, Portugal has approximately 3.5 hospital beds per 1000 people.
- There are about 5,6 doctors per 1000 people in Portugal.
Reasons to Conduct Clinical Trials in Portugal
- High-Quality Data from a solid track record of reliable clinical research;
- Diverse Patient Pool with over 10 million residents offering substantial recruitment potential;
- Eager Patient Participation as patients view trials as opportunities to access new therapies;
- Skilled Clinical Teams comprising highly skilled, qualified, and motivated investigators and staff;
- Advanced Medical Infrastructure with a network of specialized facilities in major urban areas like Lisbon and Porto;
- Cost Efficiency in clinical trials, achieved through efficient recruitment and moderate operational costs;
- EU Membership since 1986 ensures regulatory alignment and facilitates clinical trial approvals;
- Access to European Patient Database which enhances the scope and effectiveness of clinical trials;
- Innovative Frameworks used by institutions like 2CA-Braga leading to high recruitment rates and rapid trial initiation;
- Regulatory Excellence with a streamlined national system that simplifies the approval process.
Snapshot of Portugal’s Clinical Trials
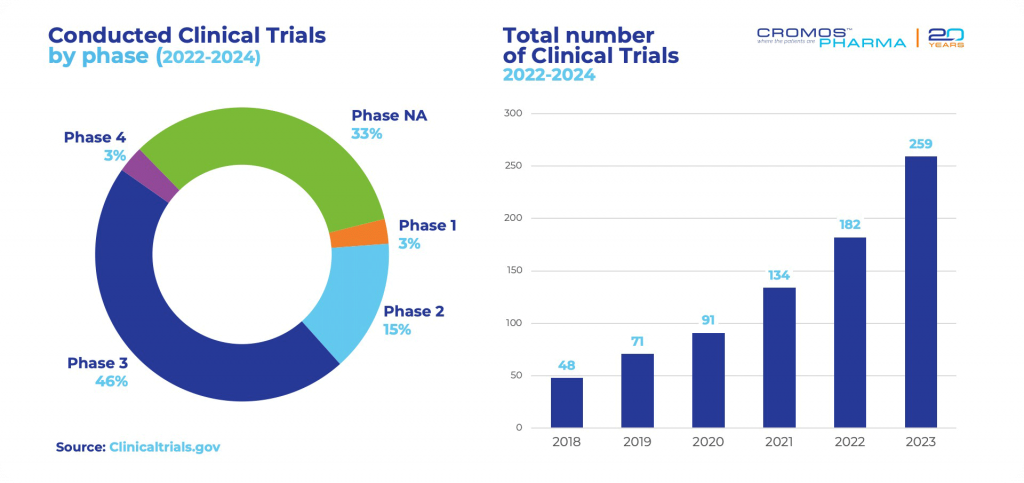
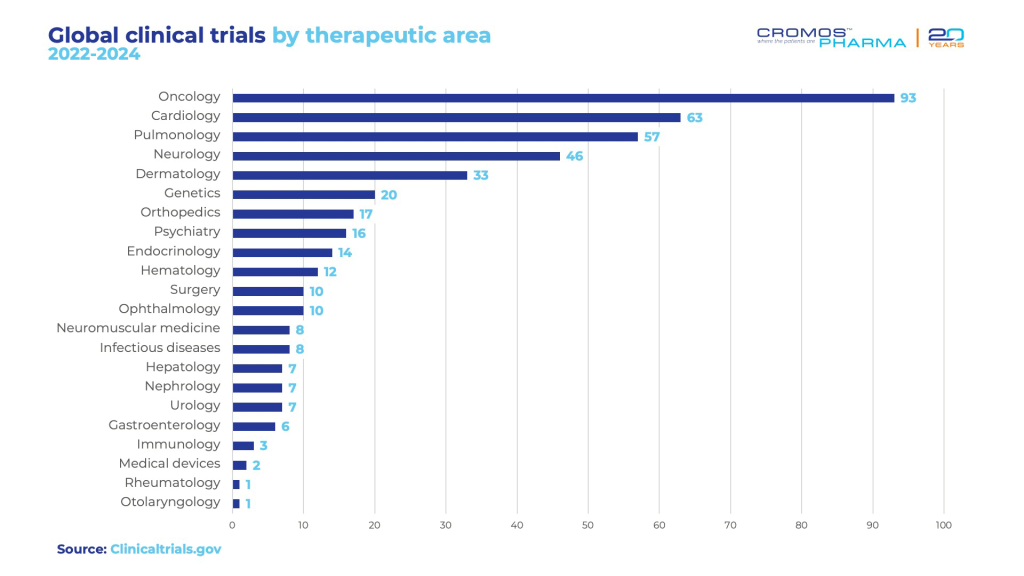
Since 2018, there has been a significant rise in the number of studies conducted in Portugal, and despite the challenges posed by COVID-19, this upward trend continues. Starting in 2022, 441 research projects have been initiated. Recent data from clinicaltrials.gov reveals that the majority of active clinical trials are concentrated in the field of oncology, with 93 trials currently underway. Cardiology follows, with 63 active trials, and pulmonology with 57 ongoing studies. Most of the research is concentrated in Phase 3, which includes 204 of these trials.
Portugal: Quick Facts
- Regulatory Approval Process
Applications for clinical trials in Portugal must be submitted through the European Union’s Clinical Trials Information System (CTIS). The review process for both Part I (scientific) and Part II (ethical) documents typically concludes within 60 days, ensuring a streamlined approval pathway.
- Agreements with Sites and Investigators
In Portugal, clinical trials typically necessitate a tripartite agreement involving the sponsor, the site, and the Principal Investigator (PI). Using a standardized Clinical Trial Agreement template tailored for Portugal can streamline the negotiation process. Moreover, some sites possess their own bilingual templates in English and Portuguese, potentially simplifying procedural aspects.
- Ethics Committee Review and Approval
The national Ethics Committee performs the ethical review (Part II), with all submissions also channeled through the CTIS. This centralized process helps maintain consistency across the board.
- Preferred Trial Site Locations
Key clinical trial sites are often located in Portugal’s major cities such as Lisbon, Porto, and Coimbra, where there is a concentration of medical expertise and infrastructure. Additionally, cities such as Guimarães, Braga, and Faro have developed well-equipped clinical trial facilities, further expanding the country’s capacity for conducting high-quality research across different regions. These emerging sites offer a valuable opportunity for broader participant recruitment and contribute to the geographic diversity of clinical trials in Portugal.
- Legal Entity Requirements
Non-EU sponsors must designate an EU legal representative. Submissions, especially those involving the Ethics Committee, must include documents in Portuguese or both Portuguese and English, highlighting the need for local linguistic expertise.
- Quality Assurance
A Qualified Person (QP) Declaration is required to affirm that the drug manufacturing site complies with EU Good Manufacturing Practices (GMP).
- Documents Needing Special Attention
The Informed Consent Form (ICF) should be in Portuguese and tailored with country-specific information to meet local regulatory requirements.
- Official Language for Documentation
While essential documentation may be submitted in English, patient-related documents, and protocol synopses must be translated into Portuguese. Labels are only required in Portuguese if the IMP is handled directly by the subject. For instance, if the IMP is administered intravenously, the translation is not needed.
- Patient Insurance
Clinical trials in Portugal require a global insurance agreement aligned with a local legal entity. It is also advisable to have a Portuguese-speaking local contact available to assist patients.
Regulatory Environment for Clinical Trials in Portugal
As of January 31, 2022, the European Union’s Clinical Trials Regulation No 536/2014 (CTR) came into effect, replacing the previous Clinical Trials Directive (2001/20/EC). This regulation standardizes the submission and assessment of clinical trials across all EU and European Economic Area (EEA) Member States, including Portugal.
- Submission under CTR via CTIS
From January 31, 2023, all new clinical trial applications (CTAs) in Portugal must be submitted through the Clinical Trials Information System (CTIS). This platform facilitates a streamlined approach for both Part I (Scientific Review) and Part II (Ethical Review) of the application dossier, which can be submitted simultaneously or sequentially.
- Document Requirements
The CTR mandates a comprehensive list of documents to be included in the submission, as detailed in Annex I of Regulation No 536/2014.
- Assessment Timeline
The general timeline from study submission to initial approval in Portugal is 60 days. This includes a 10-day validation phase, a 45-day assessment period, and a final decision phase of 5 days. Both parts of the application can be assessed in parallel within 45 calendar days, extending up to 76 calendar days if additional information is requested.
Portugal is increasingly recognized as an attractive location for conducting clinical trials, thanks to its alignment with EU regulatory standards, a diverse patient population, advanced medical infrastructure, skilled clinical professionals, and cost-effectiveness. The country’s commitment to regulatory excellence and streamlined procedures makes it an ideal setting for biotech advancements, providing substantial opportunities for biotech professionals to conduct impactful research.
For professionals looking to navigate the clinical trial landscape in Portugal, engaging with the CTIS and understanding the specifics of the CTR are crucial steps. Portugal’s integration into the broader EU framework ensures a reliable and efficient environment for clinical research, contributing significantly to global advancements in biotech and patient care.
Cromos Pharma – Your Strategic Ally in International Clinical Research
Cromos Pharma prides itself on its competent local team in Portugal, which excels in smoothly handling regulatory and contracting issues to ensure rapid initiation of studies. Our team comprises highly trained and experienced professionals who ensure that each trial we oversee delivers superior data quality and reliable outcomes.
Our company integrates global expertise with in-depth local knowledge, leading to outstanding patient recruitment rates. Remarkably, our team has met or exceeded enrollment timelines in 95% of the trials we have facilitated.
If you’re considering conducting clinical trials in Portugal or any other location where Cromos Pharma operates, our dedicated team is ready to assist with any questions you might have.