Celebrating Rare Disease Day 2024
Today, on Rare Disease Day, we emphasize the importance of bringing attention to conditions that, though rare, affect millions around the world. A striking total of over 440 million people globally face these diseases, underlining the critical need for increased advocacy and decisive action. Currently, the world benefits from the availability of over 500 drugs targeting rare diseases, with a significant portion, over 45%, being biologics. This points to a growing trend among biotech firms to broaden their offerings of biologic treatments for these ailments.
In the U.S., over 30 million individuals are dealing with rare diseases, with Europe not far behind, hosting over 26 million cases. These figures starkly reveal the extensive impact rare diseases have on communities across the globe.
The Challenge of Clinical Trials
The path to conducting clinical trials for rare diseases is fraught with distinct challenges:
- The process takes longer due to the limited number of treatment centers for these diseases.
- Choosing the right location is difficult because rare diseases are more prevalent in specific regions.
- Studies can only be conducted at sites where patients are already receiving treatment, which restricts the options available.
- Study sites need to excel in patient-centric services, such as assisting with travel arrangements and using technology for appointment scheduling, to improve the experience for participants.
- Those overseeing the studies need specialized training due to the unique care requirements of rare diseases.
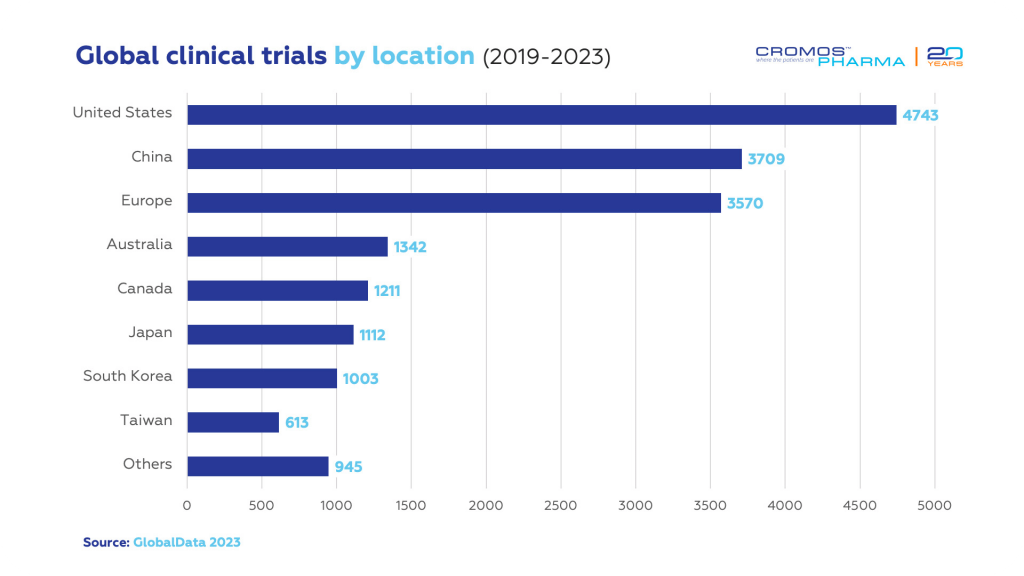
The Global Landscape of Clinical Trials
Despite the hurdles, the frequency of clinical trials targeting rare diseases has seen a significant rise from 2019 to 2023, with over 16,500 trials initiated. The U.S. and Europe lead this charge, representing 53% of all such trials, with China, Australia, Canada, and Japan also making notable contributions.
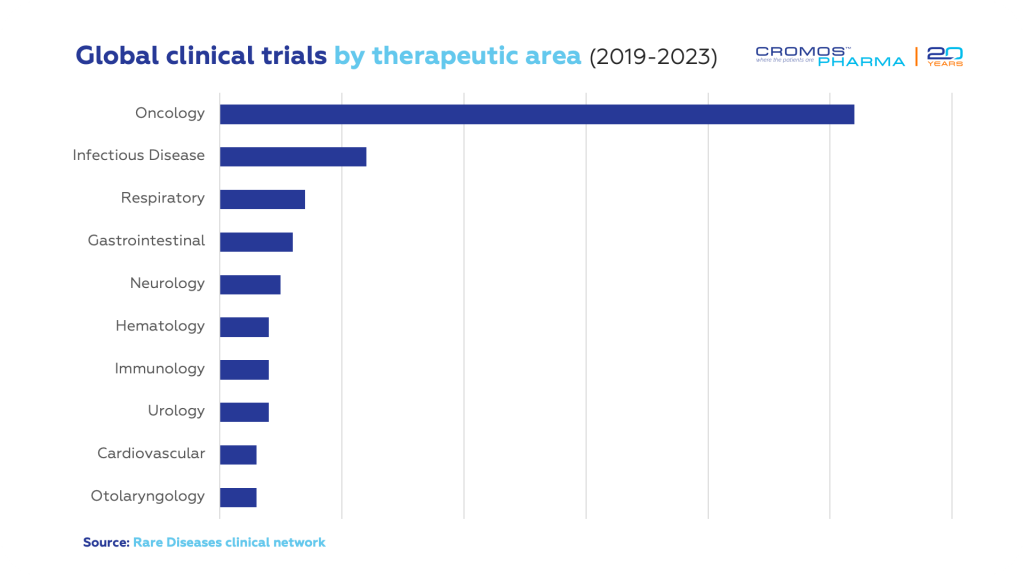
Therapeutic Areas in Focus
Oncology leads the therapeutic areas in rare disease clinical trials, particularly with research on rare cancer types, accounting for a substantial 34%. The increase in rare cancer drug approvals, more oncology trials, and a heightened focus on advancing treatments for rare cancers fuel this growth. Other important fields include infectious diseases, CNS disorders, and hematological disorders.
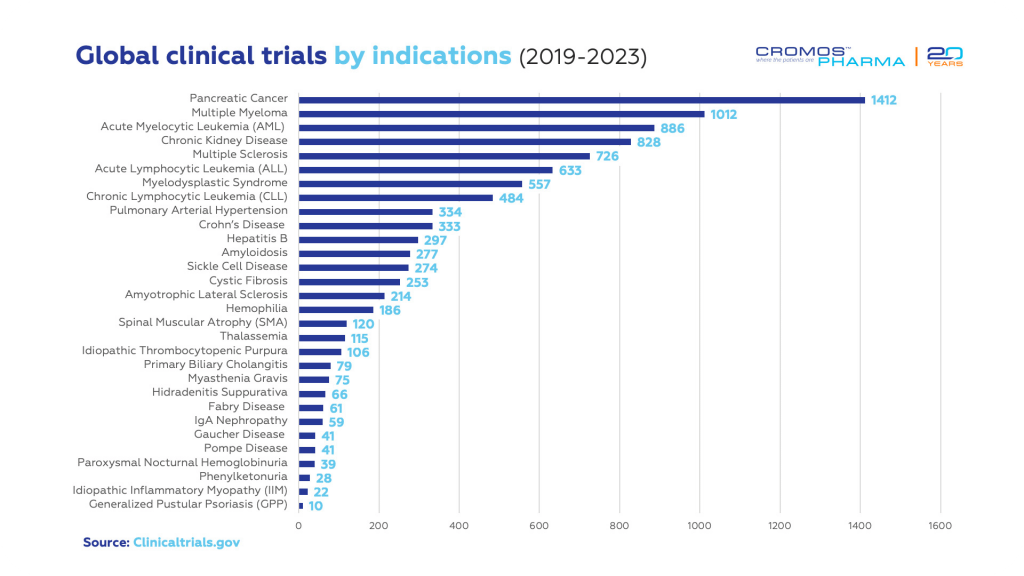
Clinical Trials by Indications
Since 2019, particular conditions have been the focus of rare disease trials, with pancreatic cancer, multiple myeloma, AML, MDS, SCD, and CLL being significant in the U.S., and MS, CF, and MG heavily studied in Europe.
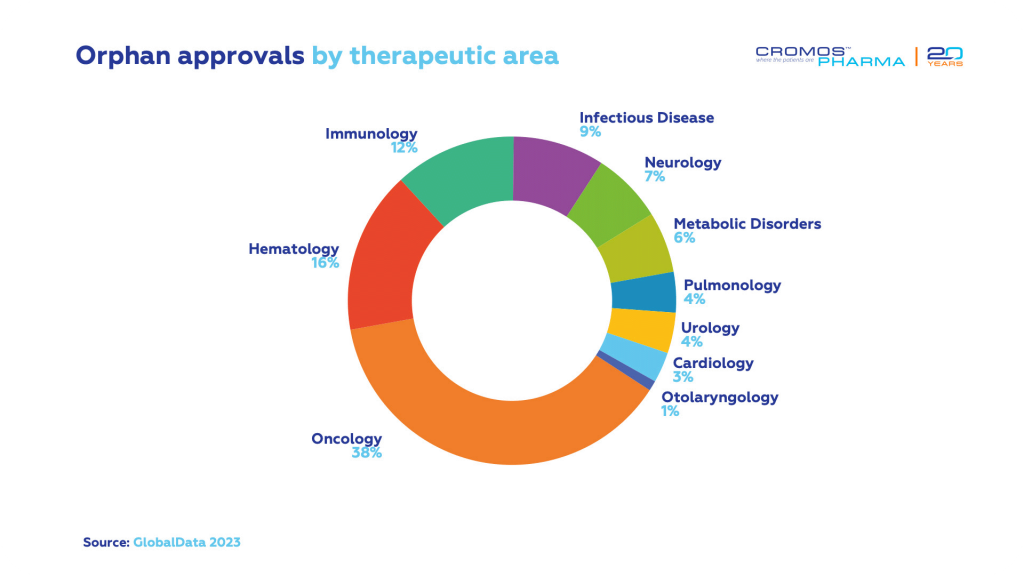
Orphan Drug Approvals
Orphan drug approvals mainly target oncology, hematology, and immunology, with remarkable progress also seen in drug development for infectious diseases, neurologic conditions, and metabolic disorders.
Advancing Rare Disease Research
The last five years have seen a significant surge in venture funding for rare disease R&D in the U.S., with over 1000 acquisitions totaling $50 billion. This demonstrates a strong commitment to advancing therapies for rare diseases and highlights the growing momentum in clinical research for these conditions.
Cromos Pharma’s Role
Cromos Pharma is committed to assisting pharmaceutical and biotech companies in developing treatments for rare diseases. With over 20 years of experience and a hand in over 30 Phase II-IV studies, we prioritize our sponsors’ goals and strive to make a meaningful difference in the lives of those affected by rare diseases. Our team of seasoned medical and regulatory experts provides a comprehensive approach to navigating the complexities of rare disease research, aiming to accelerate the development of treatments for these challenging conditions. Let Cromos Pharma be your partner in pushing forward rare disease research.