
Clinical Research Focus. 20th Edition
After Receiving First GMO Clinical Trial Approval in Georgia, Cromos Pharma Reflects on Complex International Regulatory Landscape for GMO Research
Cromos Pharma has received the groundbreaking approval to conduct the first Genetically Modified Organism (GMO) clinical trial in Georgia. This pioneering accomplishment not only underscores the company’s commitment to scientific advancement but also prompts a comprehensive reflection of the intricate regulatory landscape governing GMO trials in the US and EU.
Please follow the link to learn more.
FDA Approves First Biosimilar to Treat Multiple Sclerosis
 The U.S. Food and Drug Administration has granted approval to Tyruko (natalizumab-sztn) to Sandoz Inc., marking it as the inaugural biosimilar to Tysabri (natalizumab) injection. This approval pertains to its use in treating adults diagnosed with relapsing forms of multiple sclerosis (MS). Additionally, Tyruko shares the same indication as Tysabri, which involves initiating and sustaining clinical response and remission in adult patients suffering from moderately to severely active Crohn’s Disease (CD). Such patients must either have had an insufficient response to conventional CD therapies or be unable to tolerate them, including TNF-α (tumor necrosis factor-alpha) inhibitors.
The U.S. Food and Drug Administration has granted approval to Tyruko (natalizumab-sztn) to Sandoz Inc., marking it as the inaugural biosimilar to Tysabri (natalizumab) injection. This approval pertains to its use in treating adults diagnosed with relapsing forms of multiple sclerosis (MS). Additionally, Tyruko shares the same indication as Tysabri, which involves initiating and sustaining clinical response and remission in adult patients suffering from moderately to severely active Crohn’s Disease (CD). Such patients must either have had an insufficient response to conventional CD therapies or be unable to tolerate them, including TNF-α (tumor necrosis factor-alpha) inhibitors.
Please follow the link to learn more.
NIH-Funded Study Supports Use of ECMO For Critically Ill Patients with Obesity
 A study supported by the National Institutes of Health suggests that obese adults undergoing intensive care for respiratory failure may benefit from the use of extracorporeal membrane oxygenation (ECMO), an advanced form of respiratory support. Previously, concerns existed about using ECMO in obese patients due to potential treatment complications. However, the latest findings, published in the American Journal of Respiratory and Critical Care Medicine, indicate that obese patients receiving ECMO for acute respiratory distress syndrome (ARDS) experienced lower mortality rates compared to non-obese patients. These results raise new questions about the impact of obesity on critical illness outcomes and provide valuable insights into evidence-based treatment strategies.
A study supported by the National Institutes of Health suggests that obese adults undergoing intensive care for respiratory failure may benefit from the use of extracorporeal membrane oxygenation (ECMO), an advanced form of respiratory support. Previously, concerns existed about using ECMO in obese patients due to potential treatment complications. However, the latest findings, published in the American Journal of Respiratory and Critical Care Medicine, indicate that obese patients receiving ECMO for acute respiratory distress syndrome (ARDS) experienced lower mortality rates compared to non-obese patients. These results raise new questions about the impact of obesity on critical illness outcomes and provide valuable insights into evidence-based treatment strategies.
Please follow the link to learn more.
EMA Releases a Reflection Paper on the Role of AI in Drug Development
 The European Medicines Agency (EMA) has released a reflection paper outlining its thoughts on integrating artificial intelligence (AI) in supporting the progression, oversight, and utilization of human and veterinary medicines. This document covers AI’s application from drug discovery to post-authorization.
The European Medicines Agency (EMA) has released a reflection paper outlining its thoughts on integrating artificial intelligence (AI) in supporting the progression, oversight, and utilization of human and veterinary medicines. This document covers AI’s application from drug discovery to post-authorization.
Please follow the link to learn more.
The ALINA Study on Alectinib Achieves Its Primary DFS Endpoint in ALK-Positive NSCLC
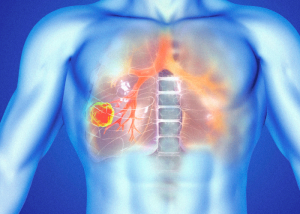 Roche has presented initial evidence suggesting that its oral inhibitor targeting anaplastic lymphoma kinase (ALK) may play a pivotal role in treating early-stage non-small cell lung cancer (NSCLC).
Roche has presented initial evidence suggesting that its oral inhibitor targeting anaplastic lymphoma kinase (ALK) may play a pivotal role in treating early-stage non-small cell lung cancer (NSCLC).
In a Phase III trial, Roche’s Alecensa® (alectinib) has become the first ALK inhibitor to show a reduction in the risk of disease recurrence or mortality in patients with early-stage ALK-positive NSCLC.
Please follow the link to learn more.
EMA Follows FDA in Approving First RSV Vaccine to Protect Infants and Older Adults
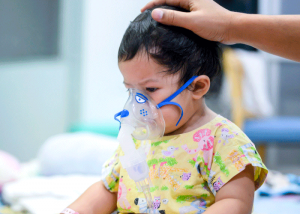 Following recent FDA approvals, the European Medicines Agency has issued a recommendation to grant marketing authorization within the European Union for Abrysvo, a vaccine designed to safeguard against respiratory syncytial virus (RSV)-related diseases. Abrysvo stands out as the first RSV vaccine intended for passive immunization of infants from birth up to 6 months of age, administered to mothers during pregnancy. Additionally, this vaccine is indicated for active immunization in adults aged 60 years and older.
Following recent FDA approvals, the European Medicines Agency has issued a recommendation to grant marketing authorization within the European Union for Abrysvo, a vaccine designed to safeguard against respiratory syncytial virus (RSV)-related diseases. Abrysvo stands out as the first RSV vaccine intended for passive immunization of infants from birth up to 6 months of age, administered to mothers during pregnancy. Additionally, this vaccine is indicated for active immunization in adults aged 60 years and older.
RSV is a prevalent respiratory virus typically resulting in mild, cold-like symptoms. However, it can pose severe risks to both children and older adults. In children, RSV ranks as a leading cause of pediatric hospitalizations in Europe, potentially leading to bronchiolitis, pneumonia, and fatal respiratory distress.
Please follow the link to learn more.
Advancing Clinical Trial Diversity: A Step Towards Equitable Healthcare
 The lack of diversity in clinical trials has long been a concern, leading to adverse side effects and health disparities among various racial and ethnic groups, as well as women. Historically, clinical trials have disproportionately included white men as participants, which limited our understanding of how drugs and treatments affect different populations.
The lack of diversity in clinical trials has long been a concern, leading to adverse side effects and health disparities among various racial and ethnic groups, as well as women. Historically, clinical trials have disproportionately included white men as participants, which limited our understanding of how drugs and treatments affect different populations.
This article explores the reasons for the lack of diversity in clinical trials and examines the steps being taken to promote inclusivity, containing revised inclusion criteria, outreach, diverse recruitment strategies, data analysis, and updated guidelines and regulations.
Please follow the link to learn more






























