
Clinical Trials in Kazakhstan
There are many benefits to conducting clinical research in Kazakhstan, its strategic location being one of them. Kazakhstan is a transcontinental country located between Europe and Central Asia; it’s health demographics draw influence from both continents. This is one reason why more and more healthcare organizations are becoming interested in this nation as a venue for conducting their clinical studies.
Cromos Pharma began its operations in Kazakhstan in 2017. Our contract research organization, headquartered in the United States, initially conducted Phase III trials in Kazakhstan, and then proceeded to expand the scope of its medical studies in the region.
Country Overview
According to the latest World Bank data, the Republic of Kazakhstan has a total population of approximately 19 million people, growing at a rate of 1.3% per annum. The main population of Kazakhstan is Kazakhs, one of the Turkic peoples of Central Asia. According to Kazakhstan’s Statistics Committee, about 11.5 million Kazakhs currently reside in Kazakhstan. The capital of the country is Astana. Other principal cities are Almaty, Karaganda, Pavlodar, Aktobe, Atyrau, and Shymkent.
Health and Pharmaceutical Industry Overview
- Kazakhstan is a relatively youthful country with only 7.9 percent of the population being 65 years or older. The median age in Kazakhstan 30.9 years. The largest age group is 25-54 years old, which makes up 42.3 percent of the populous. The average life expectancy in Kazakhstan is 71 years.
- While the pharmaceutical industry in the country only accounts for 0.45% of its GDP, the sector has grown by 47% during 2021, and is projected to continue expanding at a similar rate in the subsequent years.
Brief List of Benefits to Conducting Clinical Trials in Kazakhstan
- Treatment-naïve and ethnically diverse patient population motivated to find a higher standard of care
- Low number of studies per patient
- Moderate investigator fees
- State-sponsored healthcare that lowers the financial burden for the Sponsor
- Most of the population is concentrated in major cities, where the sites are usually well-equipped healthcare centers
- Local insurance is not required if the global insurance includes Kazakhstan
Kazakhstan has a beneficial competitive landscape for clinical trials, with a population of more than 19 million people. There are currently 52 ongoing trials, creating significant potential for rapid recruitment in future studies.
A Snapshot of Clinical Trials in Kazakhstan
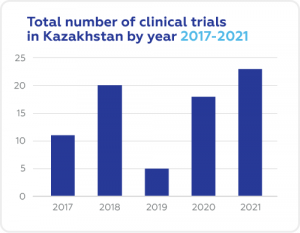
Cromos Pharma in Kazakhstan
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest amount of time possible. We recruit only highly educated and experienced staff, which assures that each trial that we conduct in Kazakhstan produces exceptional data quality and reliable results.
Summary
Kazakhstan continues to improve various facets of its public healthcare system. The government is very supportive of clinical trials, modifying policies, and providing assistance to its residents who are willing to volunteer and take part in clinical research. With constant development and continuous investments, Kazakhstan is bound to become one of the most important locations for clinical trials in Central Asia.
About Cromos Pharma
Cromos Pharma is a US-based, international contract research organization delivering fully integrated clinical research solutions, in all trial phases, across a wide range of therapeutic indications. Our expert team, comprised of 95% MDs, has extensive expertise in study design, medical writing, regulatory affairs, site management, patient recruitment and data management.
Cromos Pharma has experience in delivering success in a wide range of trial types, from biosimilars and generics, to successfully managing trials of novel therapeutics in a wide range of clinical indications. Our team provides full-service solutions to international pharma and biotech companies in high-recruiting regions, assuring exceptional data quality. Cromos Pharma combines global expertise with in-depth experience and knowledge in the US, Central and Eastern Europe, Central Asia, Republic of Georgia, and Türkiye to offer exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of conducted trials.
We provide accelerated study start-up timelines in our regions of operation. Regulatory inspections by FDA and EMA and site audits attest to the highest quality of our clinical data.
Established in 2004, Cromos Pharma has strong regional experience that is supported by a global network of offices. Its international HQ is located in Portland, Oregon, USA and its European HQ is in Dublin, Ireland.






























