
Ukraine Strives to Remain a Hub for Clinical Trials Despite Conflict
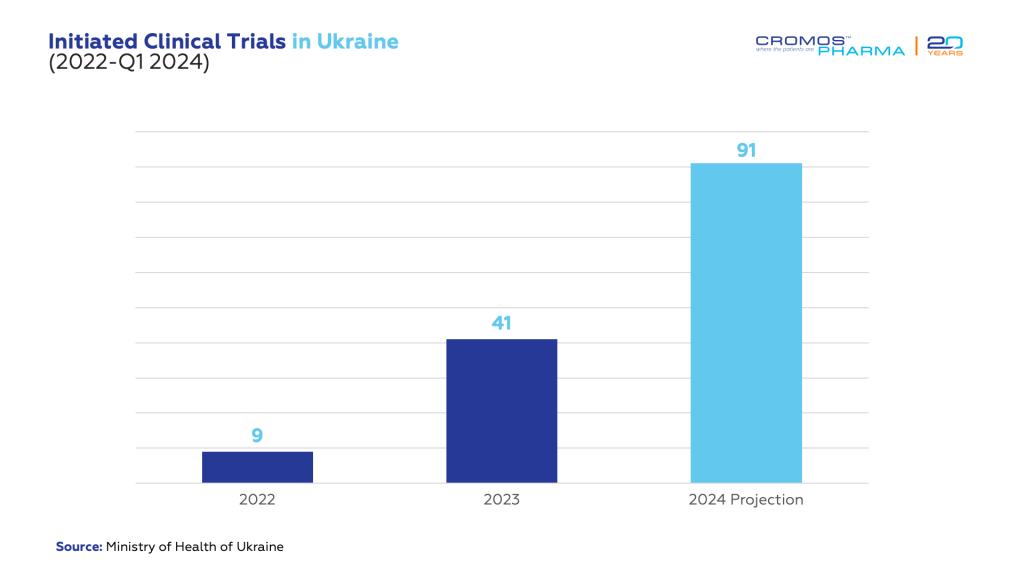
In the last couple of decades Ukraine had established itself as a thriving hub for clinical trials, especially recognized for its advancements in oncology research. Before the conflict began, the country was a beacon for international medical research, attracting increasing number of studies each year. However, the onset of the war significantly slowed this activity, with the number of new trials plummeting from around 300 annually to just 9 in 2022.
Resilience Amidst Conflict
Two years into the conflict, without a clear end in sight, the resilience and determination of Ukrainian researchers and leaders in the industry remain clear. They continue to advocate strongly for the revival of clinical trials, maintaining Ukraine’s appeal as a vital center for medical studies.
Despite these efforts, the ongoing conflict poses significant challenges, causing potential sponsors to be cautious due to concerns about the continuity of research and the integrity of data. The added complications of conducting trials in active conflict zones heighten these concerns.
Safety
Beyond the zones of direct conflict, the nation has adapted remarkably, with economic and day-to-day activities persevering – though the rhythm of life now includes the occasional air raid siren.
Cities like Kyiv, despite initial setbacks, are resurging in clinical trials. The country has managed to maintain its clinical trial infrastructure even under the challenging conditions of conflict. While the conflict is concentrated in the eastern parts of Ukraine, cities like Lviv, Ivano-Frankivsk, Khmelnytskyi, and others in the Western region continue to uphold stability, allowing the clinical trial industry and other sectors to strive towards normalcy.
Logistics and healthcare infrastructure
Sponsors considering the launch or continuation of clinical trials in Ukraine can take confidence in the country’s demonstrated capacity to sustain and manage medical supply chains amidst the current conflict. Ukrainian authorities and local organizations have effectively restructured logistics to assure the prompt and secure transport of clinical trial supplies. By rerouting shipments to arrive through alternative countries, mainly Poland, they have bypassed the constraints posed by the inoperative civil aviation system.
Furthermore, the country’s clinical trials logistics providers have demonstrated their adaptability and resourcefulness. They managed to secure storage facilities and maintained the integrity of temperature-sensitive supplies during transport.
Ukraine’s healthcare infrastructure has been strengthened with essential tools like generators, which help counter power outages. Additionally, advanced communication solutions such as Starlink terminals ensure that connectivity remains uninterrupted.
Labor force
Running clinical trials demands specialized expertise, and Ukraine’s scientific community has felt the impact. By the end of 2022, nearly one in five Ukrainian scientists had departed the country. However, some researchers who left during the early months of the conflict have now returned. The overall situation indicates that the core of specialists in Ukraine has remained intact, ensuring that the professional capacity and capability required to conduct significant research are still in place.
Patient recruitment
Ukraine’s medical system is well-organized and technologically advanced, making it a cost-effective location for clinical trials. The willingness of Ukrainians to participate in these trials provides them access to treatments that are otherwise unavailable, enhancing the appeal of conducting research in the region. This situation is particularly beneficial for pharmaceutical companies, as the often limited prior exposure of the Ukrainian population to diverse treatments simplifies the process of demonstrating the effectiveness of new medications.
What Additional Factors Should Sponsors Consider?
Despite the ongoing conflict, conducting clinical trials in Ukraine presents a unique set of benefits and opportunities that contribute to both local and global medical advancements.
- Access to Diverse Patient Populations: Ukraine’s population is diverse, offering a broad spectrum of genetic backgrounds and varying disease prevalence. During times of conflict, access to healthcare services may become limited, but this diversity remains intact. Clinical trials conducted in Ukraine can tap into this diversity, offering researchers insights into how different populations respond to medical interventions. This diversity is particularly valuable in fields such as oncology and infectious diseases, where genetic factors can influence treatment outcomes.
- Lower Operational Costs: One of the advantages of conducting clinical trials in Ukraine, even during times of conflict, is the relatively lower operational costs compared to many Western countries. Despite the challenges posed by the conflict, Ukraine still maintains a skilled workforce of healthcare professionals and researchers. Additionally, the cost of living and conducting research activities in Ukraine is generally lower than in Western European countries or the United States. This cost-effectiveness can be appealing to sponsors and researchers seeking to maximize their research budgets without compromising on quality.
- Streamlined Regulatory Processes: In recent years, Ukraine has made significant strides in aligning its regulatory framework for clinical trials with international standards. While conflicts may disrupt certain aspects of governance, Ukraine’s commitment to improving its regulatory environment for clinical research remains steadfast. The State Expert Center of the Ministry of Health of Ukraine continues to oversee the approval and oversight of clinical trials, ensuring compliance with ethical and safety standards. For sponsors and researchers, this streamlined regulatory process can expedite the initiation and conduct of clinical trials.
- Rapid Patient Recruitment: The conflict in Ukraine has unfortunately resulted in an increased burden of disease and healthcare needs. While this is undoubtedly a tragic consequence of conflict, it also presents an opportunity for clinical researchers. In some cases, there may be a rapid influx of patients seeking medical care, providing researchers with an opportunity to quickly recruit participants for clinical trials. This accelerated patient recruitment can be particularly advantageous for studies focused on acute or prevalent conditions, where timely enrollment is critical for the success of the trial.
- International Collaboration and Solidarity: During times of crisis, the global healthcare community often comes together in solidarity to address shared challenges. Conducting clinical trials in Ukraine amidst conflict fosters international collaboration and solidarity in the pursuit of scientific advancement. International sponsors, research organizations, and humanitarian agencies may collaborate with local institutions to support research efforts and improve access to healthcare services for affected populations. This collaboration not only benefits the participants of clinical trials but also strengthens research networks and capacities in Ukraine.
Clinical Trials in Ukraine in 2024
As we reflect on the current situation, we see a Ukrainian clinical trial landscape that is not merely surviving but striving towards its pre-war vitality. With 91 studies anticipated this year, there is a palpable sense of optimism. Despite the conflict, there has been significant progress, with a resurgence of studies underway. Today, the nation is preparing for a sizable increase in clinical trials, including the continued presence of prominent international pharmaceutical entities such as Pfizer, AstraZeneca, UCB, Roche, AbbVie who have maintained their operations within Ukraine.
 In conclusion, while conducting clinical trials in Ukraine during times of conflict presents its share of challenges, it also offers unique opportunities for medical research and scientific collaboration. By leveraging the country’s diverse patient populations, cost-effectiveness, streamlined regulatory processes, rapid patient recruitment, and fostering international collaboration, clinical researchers can make meaningful contributions to medical science and improve healthcare outcomes for populations affected by conflict.
In conclusion, while conducting clinical trials in Ukraine during times of conflict presents its share of challenges, it also offers unique opportunities for medical research and scientific collaboration. By leveraging the country’s diverse patient populations, cost-effectiveness, streamlined regulatory processes, rapid patient recruitment, and fostering international collaboration, clinical researchers can make meaningful contributions to medical science and improve healthcare outcomes for populations affected by conflict.
Cromos Pharma in Ukraine
Since establishing its Kyiv office two years later in 2008, Cromos Pharma has been at the forefront of clinical research in Ukraine. Over the years, our Ukrainian team has garnered commendable achievements for global sponsors, underpinned by its vast expertise and dedication.
Cromos Pharma excels in conducting a diverse array of clinical trials, ranging from biosimilars and generics to pioneering novel therapeutics across various medical indications. Our team is adept at providing comprehensive service solutions to international pharmaceutical and biotechnology companies, particularly in regions known for high recruitment rates, thereby ensuring stellar data quality. With a blend of global acumen and deep-rooted experience spanning the US, Central and Eastern Europe, Central Asia, the Republic of Georgia, and Türkiye, Cromos Pharma has mastered the art of rapid patient recruitment while maintaining exceptional data quality. Impressively, our team has met or even shortened enrollment timelines in 95% of the trials we’ve conducted.
Should you have any inquiries or wish to explore how Cromos Pharma can enhance your next clinical program in Ukraine, we warmly invite you to reach out via email at inquiry@cromospharma.com. We are here to advance your research with precision and care.






























