Сlinical trials in Georgia – Country Profile 2022
CROMOS PHARMA IN GEORGIA
Cromos Pharma began managing clinical research in Georgia in 2013. Since then our Tbilisi-based operation has grown exponentially, managing numerous successful regional and global studies in a broad range of therapeutic areas.
Georgia is regarded as a hidden gem for those interested in initiating novel studies and rescue clinical trials. It offers international Sponsors significant benefits including: a rapid regulatory approval process, significant naïve patient populations in a range of therapeutic areas, skilled medical personnel, and a conducive economic environment for doing business.
ABOUT GEORGIA
- Georgia is a rapidly developing country with a unique location at the crossroads of Eastern Europe and Western Asia – sharing borders with Russia, Türkiye, Armenia, and Azerbaijan.
- At the end of 2021, the population of Georgia was 3.7 million, the capital and largest city is Tblisi, and the country’s estimated GDP is $15.8 billion.
- It declared its independence from the Soviet Union in 1991 and since 2003 has embarked on an impressive program of economic and governance reform. Between 2010 and 2019, Georgia’s GDP per capita increased at an average annual rate of 4.8 percent. This combined with a system of social economic transfers allowed the country to nearly halve its poverty rate from 37.4 percent in 2007 to 20.1 percent in 2018 thus drastically improving living conditions.
- Additional “deep reforms” to economic management and governance have resulted in Georgia being declared a “star reformer”3 by the World Bank in 2018. These reforms have also paved the way for closer economic ties with the European Union including the signing of a Deep and Comprehensive Free Trade Area preferential trade regime. Free trade deals with the EU and China combined with robust economic growth, have resulted in Georgia gaining a reputation abroad as a good place to do business.
TOP REASONS TO CONDUCT YOUR CLINICAL TRIALS IN GEORGIA
- Lightning fast approval process. Georgia’s regulatory approval process takes on average about two months.
- Georgia’s rapidly growing study-related workforce is well experienced In international, ICH GCP-compliant clinical trials.
- Georgia’s investment in the healthcare sector over the last decade has increased the number of medical facilities and in-patient beds.
- Most hospitals are well-equipped with staff eager to participate in clinical research.
- Investigators and their patients are highly motivated to participate in trials as a way of accessing novel therapies not yet available through the national health system.
- Good recruitment potential due to an ethnically diverse population with treatment- naive patients in a broad range of therapeutic areas.
- The cost of conducting clinical research is much lower when compared to the US or Western Europe.
- Economic and political reforms have made Georgia an attractive and welcoming destination for international companies seeking to do business there.
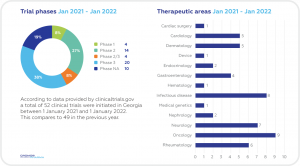
A SNAPSHOT OF CLINICAL TRIALS IN GEORGIA
THE ADVANTAGES OF CARRYING OUT CLINICAL TRIALS IN GEORGIA
Despite its relatively small size, Georgia boasts significant advantages for Sponsors seeking to carry out new trials and also to increase enrollment in ongoing projects. A high proportion of its ethnically diverse population is treatment-naïve in a wide range of therapeutic areas. A program of impressive reforms and a growing economy in recent years have increased its overall attractiveness for foreign companies and boosted its reputation as a good place in which to do business. Georgia has also made substantial progress in reforming its health system, including increased investment in healthcare facilities and programs as well as implementing a universal healthcare system (UHC) in 2013. There has been a steady increase in the number of physicians in the country since 20064 and considerable improvements in health data collection.
General information about clinical trial approval process in Georgia
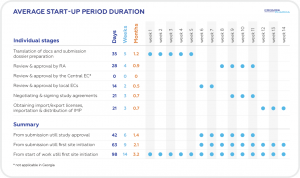
Georgia boasts one of the shortest regulatory approval timelines in Europe (see Table below for average start up duration, delineated by specific task/action).
Rapid regulatory approval process
In addition to these factors and of particular relevance to Sponsors seeking to initiate clinical trials is the impressively rapid regulatory study approval process (<2 months from submission to study start). This rapid start-up timeline, combined with a pool of skilled and professional medical staff, full compliance with ICH GCP standards and a solid reputation for producing high quality results, make Georgia a very
attractive prospect for Sponsors.
Skilled clinical professionals and strong healthcare infrastructure
Georgia has a well-educated clinical workforce with experience in ICH GCP-compliant clinical trials. There has been renewed investment in the health system in the last decade increasing the number of medical facilities and in-patient beds. Most hospitals are eager to participate in trials and have established protocols for working with international Sponsors.
Positive patient attitudes to trials
In general, investigators and their patients are highly motivated to participate in trials as a way of accessing novel therapies not yet available through the UHC.
START-UP
KEY FEATURES
- Short start-up timelines.
- Approval by local Ethics Committees (local ECs) precedes approval by the Regulatory Authority (RA = LEPL Regulation Agency for Medical and Pharmaceutical Activities at Ministry of Internally Displaced Persons from the Occupied Territories, Labor, Health and Social Affairs of Georgia).
- No import-export license is required for study drug or for lab kits / bio samples.
- Insurance for clinical trial should be obtained from local insurer.
Read our PDF here.
If you would like to find out more about how Cromos Pharma can help you with your clinical trials in Georgia contact: inquiry@cromospharma.com