Poland: Europe’s New Hub for Clinical Research
Poland has rapidly become a top destination for biotechnology professionals, drawn by its dynamic setting conducive to conducting clinical trials. This growth is underpinned by the nation’s robust healthcare infrastructure, a highly skilled workforce, a supportive regulatory framework, and favorable economic conditions, all combining to create an ideal landscape for biotechnological advancements.
A key strength of Poland in the biotech field is its diverse and extensive patient population. With about 40 million residents, Poland is the sixth most populous country in the European Union. Additionally, the country’s well-organized healthcare system and the proliferation of private, specialized medical entities ensure efficient patient recruitment and follow-up, which significantly speeds up trial timelines.
Poland’s technology sector is also seeing rapid growth, establishing itself as a global hub thanks to its strategic geographical location, significant economic potential, and rich reservoir of talent. Public-private partnerships and initiatives led by entities such as the Medical Research Agency have propelled advancements. The establishment of the Polish Clinical Trials Network in 2021 has further strengthened the nation’s clinical research capabilities, focusing efforts on improving quality management across institutions and specializing in areas like oncology and hematology.
Moreover, Poland is recognized for its cost-effectiveness as a location for conducting clinical trials, with lower associated costs than many other European countries and the United States. This cost advantage, competitive operational expenses, and reduced patient recruitment costs, makes Poland an increasingly appealing destination for biotech companies looking to optimize their R&D budgets without compromising on quality.
Country Overview
Positioned in Central Europe, Poland spans approximately 313,000 square kilometers, making it the ninth-largest country on the continent. It shares borders with Germany, Czechia, Slovakia, Ukraine, Lithuania, and Belarus, with maritime proximity to Nordic countries like Sweden, Denmark, and Finland. Warsaw, the capital and largest city, boasts a population of 1.86 million within a metropolitan area of 3.27 million. Known for its rich cultural history and diverse population, Poland prides itself on a high level of English proficiency among its citizens, comparable to that of Swedes, Germans, and Finns.
Demographics
As of 2023, the population of Poland stands at approximately 39,8 million. Poland’s demographic profile shows a balanced age distribution with a median age of about 40 years, indicative of a moderately aging population. The largest demographic group, those aged 15 to 64, constitutes about 63% of the population. Moreover, over 60% of Poles lived in urban areas, with cities like Warsaw, Krakow, Lodz, and Wroclaw.
Healthcare System
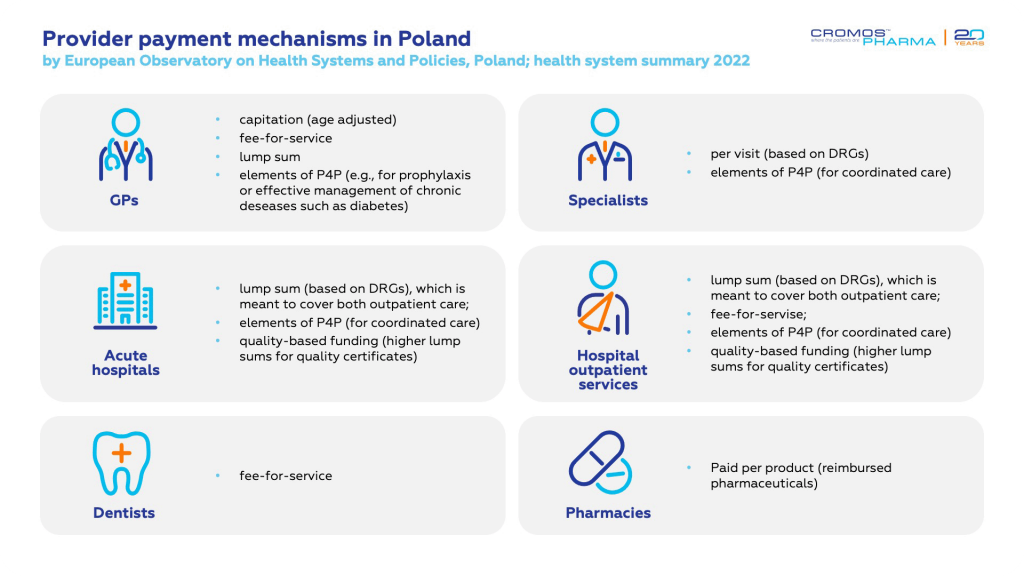
The Polish healthcare system operates on a universal social health insurance model, funded through public and private contributions. The National Health Fund (NFZ) is central to this system, managing healthcare financing, contracting with providers, and reimbursing medical services. While the primary and specialist outpatient care is often provided in private or small group practices, most hospitals are publicly owned and vary in the complexity of care they provide, from county hospitals to more specialized tertiary-level institutions.
Despite facing similar challenges to other European nations, Poland’s healthcare system has seen numerous innovative reforms, particularly following the COVID-19 pandemic. These include the implementation of digital medical records, e-prescriptions, and e-referrals. Initiatives like the National Oncology Network and the National Cardiology Network have been set up to standardize patient pathways and concentrate expertise in specialized medical fields.
The country’s healthcare infrastructure includes modern facilities and well-equipped research centers, making it an ideal environment for conducting clinical trials across various therapeutic areas. Poland’s educational system also contributes significantly to the sector, with 38 universities offering biotechnology programs as of 2019, producing a continuous supply of skilled graduates. The active biotech sector is concentrated in six major clusters across the country, enhancing Poland’s position as a significant player in global medical innovation and research.
Conducting Clinical Trials in Poland: Key Advantages
Poland provides a highly favorable environment for clinical trials, offering strategic benefits crucial for sponsors:
- Large Patient Population. With a sizeable and diverse population, Poland provides a wide pool of potential participants, spanning various demographics and medical conditions, ideal for varied clinical studies.
- Skilled Healthcare Professionals. The country is equipped with a well-developed healthcare system staffed by highly trained medical professionals and researchers, ensuring proficient management and oversight of clinical trials.
- Quality Infrastructure. Poland’s healthcare infrastructure features modern medical facilities and research centers, including hospitals and clinics equipped to international standards, facilitating high-quality clinical trials.
- Cost-Effectiveness. Clinical trials in Poland are more economical compared to many Western countries, offering significant savings on labor, infrastructure, and administrative expenses, with costs averaging 30% below those in the US.
- Efficient Regulatory Environment. The regulatory framework in Poland aligns with EU standards, supporting smooth trial initiation and execution while upholding patient safety and data integrity.
- Fast Approval Process. Polish regulatory authorities are known for their relatively swift approval timelines, enabling faster commencement of clinical trials.
- Access to Untapped Patient Cohorts. Poland provides access to diverse patient populations, including those underrepresented in research elsewhere, opening opportunities to explore novel therapies and conditions.
- Solid Track Record. The country boasts a solid track record of generating high-quality clinical research data, further strengthening its reputation as a reliable location for clinical studies.
- Strategic Geographical Location. Situated centrally within Europe, Poland offers logistical benefits for multinational clinical trials, with easy access to neighboring countries and their patient bases.
- Government Support. The Polish government actively supports research and development, particularly in clinical trials, through various funding initiatives and incentives aimed at pharmaceutical and biotechnology companies.
- Quality and Support Initiatives. Poland has implemented numerous initiatives to promote quality and support in clinical trials, including the establishment of the Polish Clinical Trials Network (PCTN) and The Sponsor Support Center (SSC).
Snapshot of Poland’s clinical trials
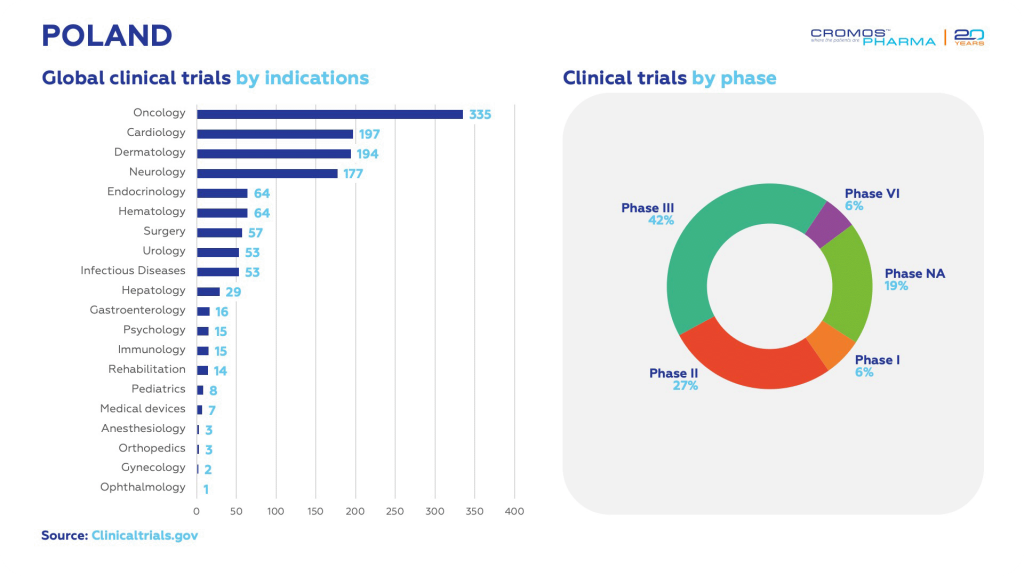
Since 2022, Poland has initiated 1 307 clinical trials, showcasing its growing significance in the global research landscape. Data from clinicaltrials.gov reveals that oncology leads with 335 trials, followed by cardiology with 197, dermatology with 194, and neurology with 177. The majority of these trials are in Phase 3, accounting for 34% of the total. Additionally, international sponsors are responsible for 73% of all ongoing clinical trials in the country, indicating a strong global interest in Poland’s clinical research capabilities.
Poland: Quick Facts
Regulatory approval process: The application must be uploaded onto the Clinical Trials Information System (CTIS). Parts I and II of the documents are assessed in parallel within 45 calendar days (60 calendar days in total for review and approval).
Agreements with sites and investigators: Usually, tripartite contract should be signed. The draft contract must be submitted and if not available, statements regarding study funding and financial operations in the study must be submitted. Having a country-specific Clinical Trial Agreement template is helpful in the negotiation process.
EC review and approval: CEC is the only ethics body involved in the review process (Part II Ethical Review). Submission should be done via Clinical Trials Information System (CTIS).
Legal entity: For non-EU Sponsors, an EU legal representative is required. CTIS application form contains sections that should be filled out in the Polish language. Submission to CEC needs to be done in either Polish or in both English and Polish. The assistance of local staff is therefore recommended.
QP Declaration / GMP certificate: Declaration from the QP stating that the manufacturing site operates in compliance with the EU GMP is required for submission.
Documents requiring special attention: Informed Consent Form (ICF) should be completed in the local language and should contain country-specific information.
Official language: Essential documentation should be submitted in English. Patient-related documents, labels, protocol synopsis must be translated into the Polish language.
Patient insurance: Global insurance agreement with the local legal entity in the country is required. A further requirement is that a local contact who speaks the Polish language should be available to assist the patient with any questions.
Regulatory Environment for Clinical Trials in Poland
On January 31, 2022, the EU Clinical Trials Regulation No 536/2014 (CTR) took effect across all EU and European Economic Area (EEA) member states, superseding the previous EU Clinical Trials Directive (2001/20/EC). As of January 31, 2023, all new Clinical Trial Applications (CTAs) in Poland and other member states must be submitted through the Clinical Trials Information System (CTIS), aligning with this regulation.
The CTR mandates that each CTIS User Administrator submits clinical trial applications in a standardized format, which includes Part I (Scientific Review documents) and Part II (Ethical Review documents). These comprehensive submissions can be made either simultaneously or sequentially, according to the specific requirements outlined in Annex I of the Clinical Trials Regulation No 536/2014.
The typical timeline for the approval process from study submission to initial approval spans approximately 60 days. This includes a 10-day validation period, a 45-day assessment phase, and a final decision phase of 5 days. It is noteworthy that Parts I and II of the application can be evaluated in parallel within a 45-day window, which may extend up to 76 days if additional information is requested.
Cromos Pharma – Your Partner in International Clinical Research
Cromos Pharma is your trusted partner in managing the regulatory and contracting processes necessary for clinical trials in Poland, guaranteeing rapid study initiation and the collection of high-quality data. Our local expertise and commitment to adhering to enrollment timelines position us as a key supporter for biotech firms looking to navigate the complex landscape of clinical trials in Poland.
Our team in Poland is composed of highly skilled and knowledgeable individuals who ensure that each trial we manage meets the highest standards of data quality and reliability. This expertise, combined with a robust infrastructure, a large and diverse patient population, favorable regulatory conditions, and overall cost-effectiveness, makes Poland an ideal environment for clinical trials.
Cromos Pharma’s global expertise, coupled with our deep local knowledge, leads to exceptional patient recruitment outcomes. We have consistently met or shortened enrollment timelines in 95% of our trials. Our extensive capabilities, strengthened by our collaborations with Phase 1 units and bioequivalence study sites, significantly enhance our ability to achieve success.
If you are considering conducting clinical trials in Poland or any other country where Cromos Pharma operates, do not hesitate to reach out. Our team is ready to assist you with any questions and support your research needs.
Engage with Cromos Pharma to experience how our dedicated team can help advance your clinical research goals effectively.