
Serbia – a land of opportunity for clinical research
The past, the present, and the future
When someone mentions Serbia in a scientific context, the name of Nicola Tesla inevitably comes to mind. But very few people know that Serbia has produced some of the truly great minds in medicine. One such scientist is Miodrag Radulovacki, who is considered to be the father of modern sleep apnea research, a disease that affects nearly a billion people worldwide.
Over the years, Serbia has become a highly reputable place to conduct clinical research. The country has actively strived towards developing a robust healthcare system and adopted full GCP compliance. There are 322 clinical trials currently occurring inside Serbia’s borders.
Increasing number of biotechnology and pharmaceutical companies are now willing to see Serbia as a potential regional hub for their business operations and thus make significant investments in the Serbian market.
Country overview
Serbia’s unique geographic location at the intersections of Central and Southeastern Europe makes it an easily accessible destination. This is also a reason why Serbia has such an ethnically diverse population. Belgrade is the capital of Serbia and is one of the oldest cities in Europe, having historical roots well into the Roman times. Its central location makes it a perfect choice for the country’s financial and cultural epicenter.
Over the past two decades, Serbia has been able to develop a thriving healthcare sector while still having a substantial treatment-naive population. Consequently, Serbia has emerged as one of the world’s favored destinations for clinical research.
Demographics and socioeconomics
The total population of Serbia is 8.6 million. Recent data shows that 59.4% of Serbia’s population resides in urban areas, including 16.1% of the people living in the Belgrade region. The socioeconomic climate is summarized as an emerging middle social class and a nation with a developing free market economy.
Due to Serbia’s resourcefulness and economic capabilities it has secured a preferential trade partnership with the United States, a preferential trade regime with the European Union and European Medicines Agency, and free trade agreements with the CEFTA and EFTA.
Considering all these factors, Serbia has abundant resources at its disposal for its clinical research industry to thrive.
Healthcare sector in Serbia
The current life expectancy in Serbia is 76, with the largest proportion of the population is between ages of 15 and 64 years. 21.3% of the population is above the age of 65. Today the number of hospital beds per 1000 inhabitants is 5.41.
Reasons to conduct clinical trials in Serbia
The country’s efforts to further strengthen and enhance its well-established clinical research sector are constantly expanding. The decision to perform clinical trials within Serbian borders is an excellent choice for several reasons:
- High recruitment rates and vast patient population
- Moderate research costs and investigator fees
- High quality standards of clinical research
- High-quality, accredited research units tailored to clinical trials
- Well-qualified, compliant, and experienced staff of GCP-certified investigators
- Enhanced regulatory framework and validated safety guidelines under Serbian law and the Medical Devices Agency
- An increasing and ever-improving business infrastructure for clinical trials, including advancing medical devices
- An opportunity to participate in clinical trials gives Serbian patients an opportunity to have access to novel biologics, which are still limited under the state-funded supply programs, furthering the motivation
- Finally, a GMP certificate is sufficient for regulatory submissions and compliance and the European Union QP Statement is not required
Also, it is essential to note that Belgrade is home to the Clinical Centre of Serbia, the country’s most reputable center for clinical trials. It is one of Europe’s most prominent clinical institutions, with over 50 research installations and 3150 beds, the largest in the European Union area.
Serbia: Quick Facts
Regulatory approval process
Submission to CEC and RA (MoH) can be performed in parallel and electronically (via a web portal called ADIS). After the CEC approval for research is obtained, the RA approval is issued 30 days later.
Agreements with sites and investigators
Agreement negotiation can be started before the study approval, but some sites require at least a CEC approval to engage.
LEC review and approval
CEC and RA (MoH) reviewing processes can be done in parallel.
Trial sites’ location
All sites are located within an approximately 350 km radius of Belgrade.
Legal entity
A local legal entity is required for submission.
QP Declaration / GMP certificate
GMP Certificate, Manufacturing Authorization, and QP release (depending on EU/non-EU manufacturing) are needed for submission, and the CoA for the actual batches to be imported into Serbia.
Documents requiring special attention
ICF and all patient materials should be customized with country-specific information. Certificates of Analysis for any product used in the study must match the actual shipments.
Official language
Essential documentation should be submitted in English. Patient-related documents, labels, and protocol synopsis must be translated into Serbian.
Patient insurance
Local Insurance is required. The Insurer and the Sponsor sign the agreement.
Useful tips
There is a strict requirement to list all Institutions and the PI names and addresses in the insurance documentation.
Snapshot of Serbia’s clinical trials
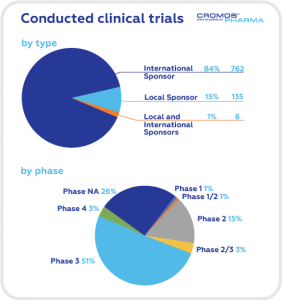
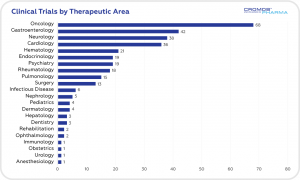
According to data obtained from clinicaltrials.gov the largest share of clinical trials is in the field of oncology (68), followed by gastroenterology (42), neurology (38), and cardiology (36). International Sponsors are responsible for 84% of ongoing trials.

Regulatory Protocols for clinical trials in Serbia
The Medicine and Medical Devices Agency of Serbia monitors the evaluation, verification, and approval processes for clinical trial applications in Serbia. This Agency is a divisional component of the Ministry of Health in Serbia and, in Serbian, is referred to as Agencija za lekove i medicinska sredstva Srbije, or ALIMS.
The Agency is responsible for implementing laws and guidelines associated with medical devices, medicinal products, cosmetics, food supply, and biologics. It is also responsible for the efficacy and safety of all medicinal products, for oversight of controlled drugs and substances, and for approving imported medical devices.
In 2004, the Serbian government executed a revised version of its clinical trial legislation. This undertaking aimed to fill the gap between the existing system and the principles defined by the European Union Clinical Trials Directive.
Likewise, the regulatory procedure clinical trial application approval is prompt, transparent, and effective. Most studies receive approval within 80 days.
Cromos Pharma – you partner in international clinical research
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest period possible. We recruit highly educated and experienced staff that assures that each trial managed by our team in Serbia produces exceptional data quality and reliable results.
Cromos Pharma combines global expertise with in-depth local experience and knowledge that translates into exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of the conducted trials.
If you are considering conducting clinical trials in Serbia or in any other countries where Cromos Pharma is present, our team would be happy to answer any of your questions.






























