
Serbia is a Rising Star in Clinical Research
In recent years, regulatory timelines for clinical trials in Serbia have grown longer, posing challenges for some biopharma companies. However, recent trends indicate a significant reduction in these timelines, fostering optimism for sponsors and stakeholders. This positive shift underscores Serbia’s commitment to creating a conducive environment for clinical research.
A Legacy of Innovation
For centuries, Serbia has cultivated a legacy of scientific discovery and innovation. From Mihailo Petrović Alas’s groundbreaking contributions to biomedical engineering to Miodrag Radulovacki’s transformative research in sleep apnea, Serbia has consistently pushed the boundaries of medical science. Today, this tradition continues as Serbia positions itself as a nexus of clinical research excellence.
Serbia: A Strategic Advantage
Located at the crossroads of Central and Southeastern Europe, Serbia offers unmatched accessibility and diversity. Its geographic location makes it an ideal hub for regional and global clinical trials, supported by:
- A population of 6.7 million, with 63% in the productive age range of 15 to 64 years.
- A diverse demographic landscape, enabling the study of varied patient profiles.
- A growing urban population (56.3%) centered around Belgrade, the economic and cultural heart of the nation.
Country overview
Strategically located at the crossroads of Europe, Serbia offers unmatched accessibility. This is also a reason why Serbia has such an ethnically diverse population. According to the most recent United Nations World Statistics Pocketbook, Serbia’s land area covers approximately 88,444 square kilometers. Belgrade is the capital of Serbia and is one of the oldest cities in Europe, having historical roots well into the Roman times. Its central location makes it a perfect choice for the country’s financial and cultural epicenter.
Healthcare System: A Solid Foundation
Serbia’s healthcare system is built on compulsory health insurance, managed by the National Health Insurance Fund (NHIF). Most healthcare facilities are publicly owned, supplemented by a robust private sector. Recent reforms have focused on:
- Enhancing preventive care.
- Integrating health information systems.
- Aligning with international Good Clinical Practice (GCP) standards.
With modern facilities and a strong academic foundation, Serbia produces highly skilled graduates in medicine and biotechnology, strengthening its position as a destination for medical innovation.
Why Choose Serbia for Clinical Trials?
Serbia’s evolving clinical research ecosystem offers numerous advantages:
- Rapid Patient Recruitment: High recruitment rates supported by a vast patient population.
- Cost Efficiency: Moderate research costs and investigator fees.
- Regulatory Improvements: Streamlined approval processes with ALIMS and the Ethics Committee.
- High-Quality Standards: Accredited research units and GCP-certified investigators.
- Regulatory Alignment: Compliance with international safety guidelines under Serbian law.
- Infrastructure: State-of-the-art facilities like the, Europe’s largest clinical institution with over 50 research installations and 3,150 beds.
- Patient Access: Clinical trials offer Serbian patients access to novel treatments not covered by state-funded programs, increasing motivation and participation.
Clinical Research Snapshot
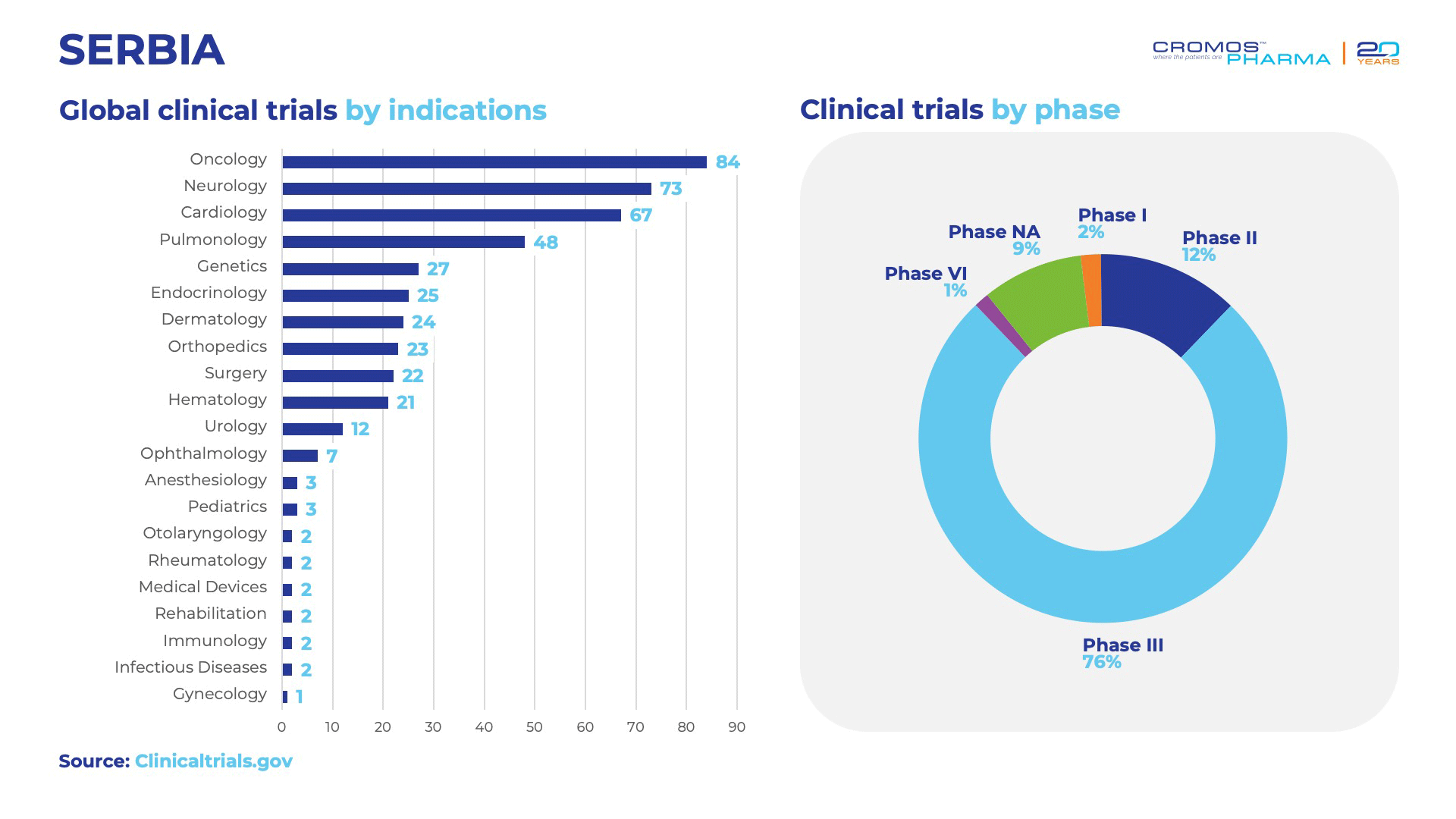
Since 2023, Serbia has launched 452 clinical trials, highlighting its increasing role in the global clinical research landscape. According to data, oncology leads with 84 trials, followed by neurology with 73, cardiology with 67, and pulmonology with 48. The majority of these studies are concentrated in Phase 3, representing a significant portion of all trials. Additionally, a large share of the trials in Serbia are sponsored by international organizations, underscoring the global interest in Serbia’s clinical research potential and capabilities.
Clinical Trials by Therapeutic Areas 2023-2024
Navigating Regulatory Protocols
The Medicines and Medical Devices Agency (ALIMS) oversees clinical trials in Serbia, ensuring compliance with international standards. Key regulatory insights include:
- Approval Process:
- Sponsors must obtain approval from ALIMS and the Ethics Committee (EC).
- The review process typically takes 60 days, with extensions for complex trials involving gene therapy or genetically modified organisms.
- Phase I trials are limited to publicly owned healthcare facilities.
- Monitoring and Compliance:
- ALIMS supervises adherence to GCP guidelines and legal standards.
- Sponsors must promptly report serious adverse events to ALIMS and the EC.
- Public Awareness:
- ALIMS promotes clinical trial transparency through educational campaigns for professionals and the general public.
Key Considerations for Sponsors
- Localized Support: A local legal entity is required to submit clinical trial applications.
- Document Requirements: All patient-related materials must be translated into Serbian, ensuring clarity and compliance.
- Insurance: Local patient insurance agreements must include all participating institutions and Principal Investigators (PIs).
- Logistics: All trial sites are located within a 350 km radius of Belgrade, simplifying coordination for multi-site studies.
Cromos Pharma: Your Partner in Serbia
With over a decade of experience, Cromos Pharma is a trusted leader in Serbia’s clinical trial landscape. Our expertise includes:
- Efficient management of regulatory and contractual processes.
- Rapid study initiation with a focus on quality and compliance.
- Consistent patient recruitment, meeting or exceeding enrollment targets in 95% of our studies.
Combining global expertise with deep local insights, Cromos Pharma ensures that your clinical trials in Serbia are executed with precision and excellence.
Partner with us to navigate Serbia’s evolving clinical research ecosystem. Contact Cromos Pharma today to learn more about how we can support your clinical trial needs.






























