
World Cancer Day 2025: How Clinical Trials Are Shaping the Future of Cancer Treatment
On this World Cancer Day, we reflect on the progress made in the fight against cancer and the challenges ahead. With over 2 million new cases diagnosed in the US in 2024 alone, cancer remains a leading cause of death.
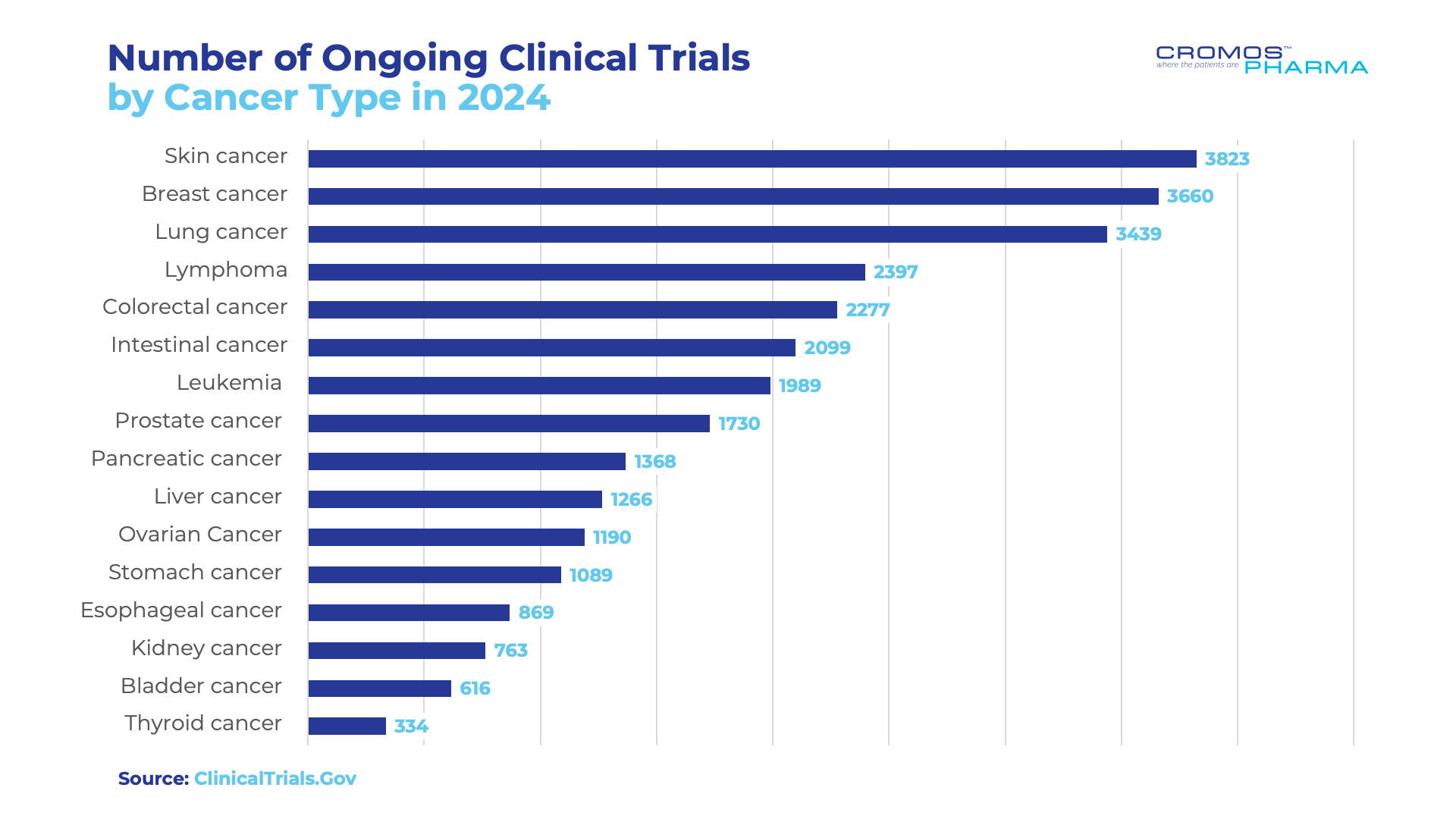
However, breakthroughs in early detection, targeted therapies, and clinical trial innovations are reshaping oncology care. Clinical trials drive these advancements, with 28,909 ongoing oncology studies worldwide exploring novel treatments across various cancer types.
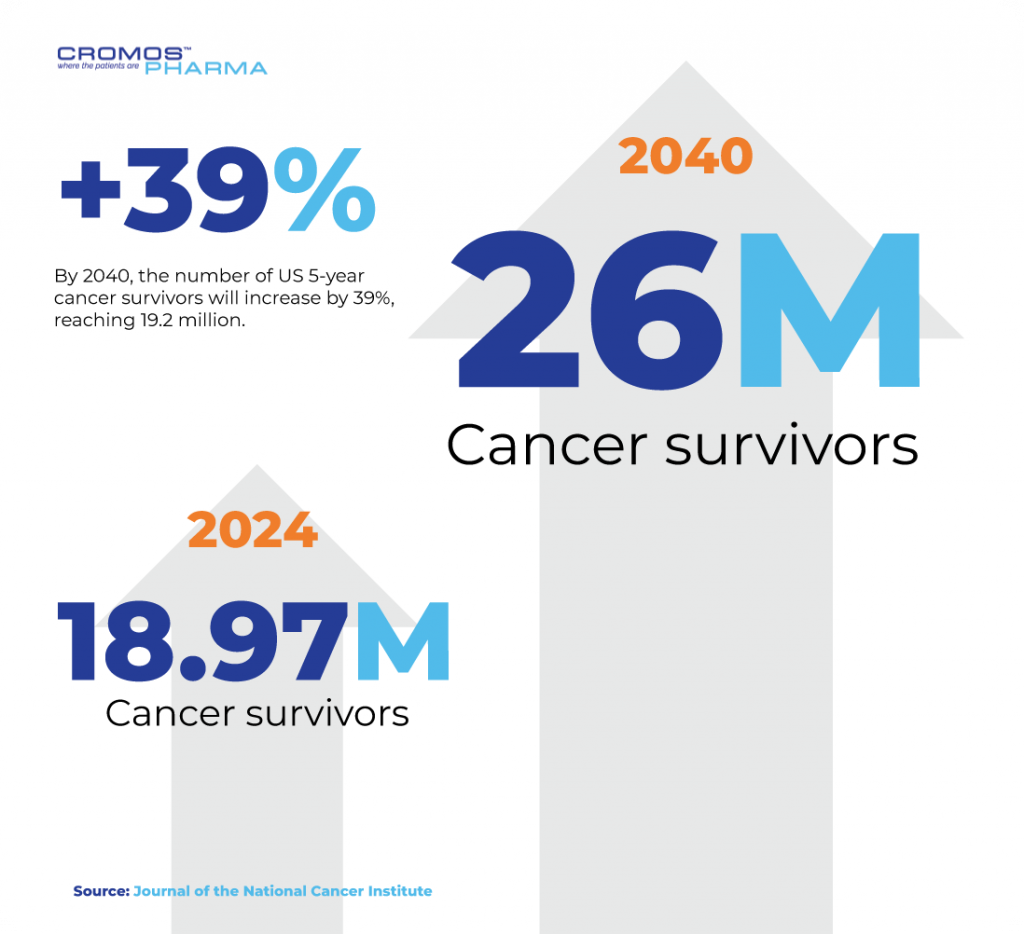
As of 2024, there were 18.97 million cancer survivors in the U.S., a figure projected to reach 26 million by 2040. Long-term survival is becoming more common, emphasizing the need for improved post-treatment care and quality-of-life initiatives. Currently, 70% of cancer survivors have lived at least five years post-diagnosis, and 11% have surpassed 25 years.
This article explores the key innovations shaping the future of oncology clinical trials, with a special focus on antibody-drug conjugates (ADCs), AI-driven drug discovery, and novel immunotherapy approaches. By understanding the most promising trials on the horizon, we can anticipate how these advancements will redefine cancer treatment and improve patient outcomes in the coming years.
1. The Evolution of Antibody-Drug Conjugates
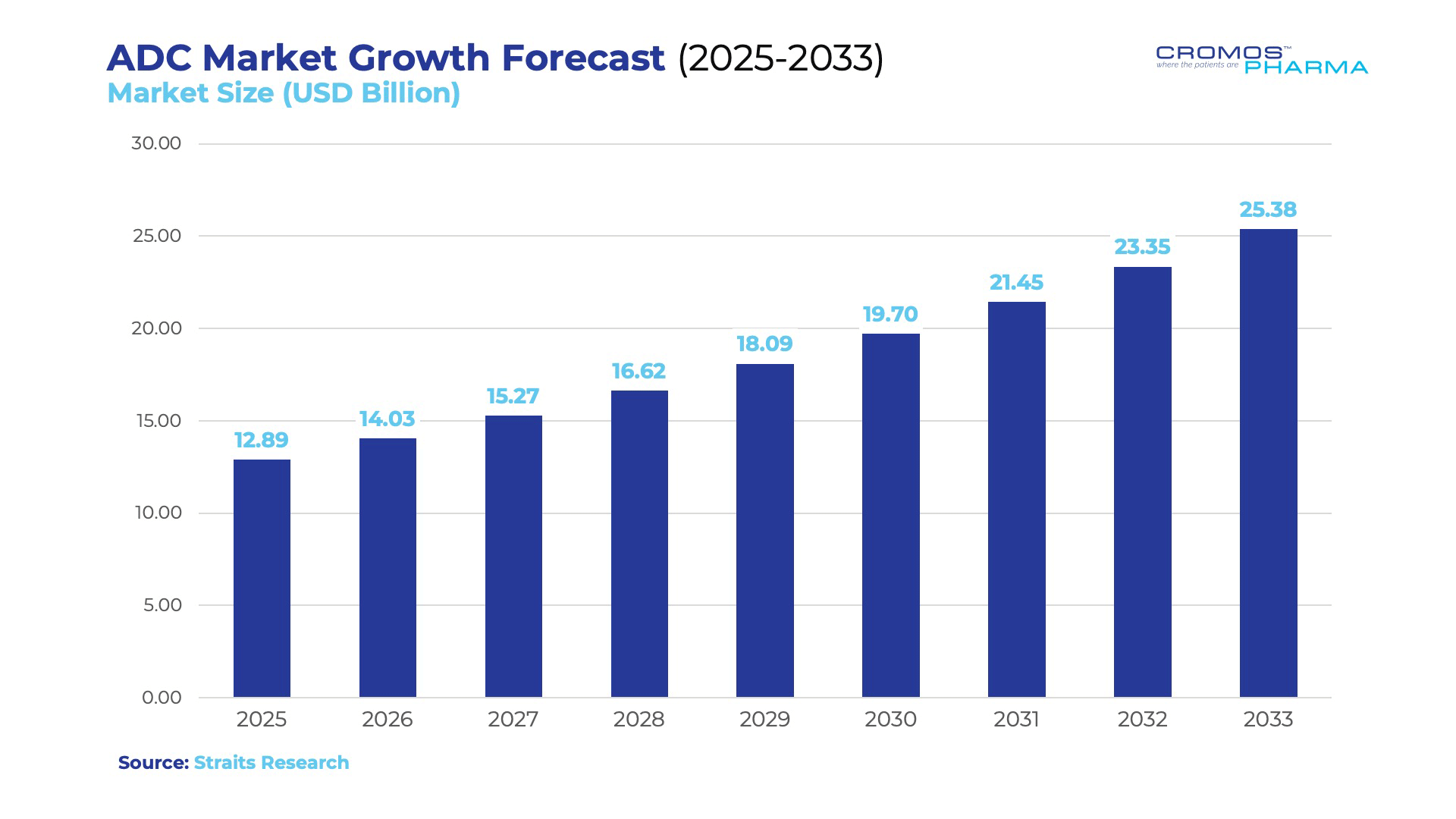
The ADC market is experiencing explosive growth, projected to surge from $11.84 billion in 2024 to $25.38 billion by 2033, driven by advancements in linker technology, payload design, and antigen targeting. As of 2024, 15 FDA-approved ADCs are revolutionizing oncology, addressing 16 different cancer types, including breast, lung, and bladder cancers, as well as hematologic malignancies.
Breakthroughs in ADC engineering—such as more stable linkers, optimized cytotoxic payloads, and improved tumor selectivity—have greatly enhanced efficacy and safety. These innovations address previous challenges like off-target toxicity and resistance, increasing ADCs’ therapeutic potential.
With higher regulatory success rates than traditional oncology drugs and a growing pipeline, ADCs represent a lower-risk yet high-reward avenue for pharmaceutical companies. While challenges remain, such as immune-related side effects and further payload optimization, the future of ADCs looks increasingly promising, with ongoing research set to push the boundaries of cancer therapy.
2. Innovations in Digitization & Decentralization of Oncology Trials
Digitization and decentralization are transforming oncology trials by improving accessibility and efficiency. Decentralized clinical trials (DCTs) eliminate geographical barriers by enabling patients to participate remotely, a crucial advancement for oncology patients requiring continuous monitoring and treatment.
Key innovations include:
- Telemedicine & Wearable Devices: Remote consultations and real-time health tracking enhance patient monitoring and engagement.
- AI & Machine Learning Integration: AI-driven platforms streamline trial design, patient recruitment, and real-time data analysis, improving efficiency.
- Home-Based Drug Administration: Programs like Penn Home Infusion Therapy and Huntsman at Home demonstrate the feasibility of delivering oncology treatments outside traditional hospital settings.
- Decentralized Trial Infrastructure: Local health facilities, labs, and mobile health applications support remote patient care while maintaining trial integrity.
The COVID-19 pandemic accelerated the adoption of remote monitoring, AI-driven recruitment, and home-based care, proving their viability for long-term implementation. By leveraging these innovations, oncology trials are becoming more inclusive, cost-effective, and patient-centric, paving the way for faster and more reliable drug development.
3. The Rise of AI in Oncology Trials
Artificial intelligence (AI) is revolutionizing oncology trials, enhancing drug discovery, diagnostics, and treatment planning. AI-powered algorithms analyze vast datasets, identifying patterns that improve patient selection, predict treatment responses, and optimize protocol design and trial monitoring .
In drug discovery, AI accelerates the identification of promising drug candidates by analyzing biological data and predicting interactions with cancer cells. Companies like Bristol-Myers Squibb (BMS) and AstraZeneca leverage AI-driven models to optimize trial designs and reduce development timelines. AstraZeneca employs over 700 AI and data science experts, using machine learning to double the speed of traditional drug discovery.
AI also improves cancer diagnostics by enhancing imaging analysis and genomic profiling. Machine learning models detect early cancer signs in medical images and identify genetic mutations linked to cancer, aiding personalized treatment strategies.
In clinical trials, AI optimizes patient recruitment and trial efficiency. Natural language processing (NLP) helps identify eligible patients, while AI-driven analytics refine study designs by predicting trial outcomes. Johnson & Johnson utilizes AI for real-time data processing, reducing reporting time and improving trial oversight.
As AI continues to advance, its role in oncology trials is expanding, making treatments more targeted, efficient, and personalized, ultimately improving patient outcomes and accelerating breakthrough therapies.
4. Advancing CAR T-Cell Therapy
CAR T-cell therapy is reshaping cancer treatment, achieving durable responses in relapsed or refractory hematologic malignancies. In 2024, the FDA expanded approvals for cilta-cel (Carvykti) and ide-cel (Abecma), allowing multiple myeloma patients to receive treatment earlier in their care journey, improving access to these life-saving therapies.
Beyond hematologic malignancies, CAR T-cell therapy is making strides in solid tumors. New research focuses on overcoming barriers like limited T-cell infiltration and the immunosuppressive tumor microenvironment. Targeting tumor-associated antigens such as HER2, GD2, and GPC3 is showing promise in glioblastoma, pancreatic, and ovarian cancer trials.
Innovations in manufacturing, including off-the-shelf allogeneic CAR T-cells, are addressing logistical challenges by eliminating the need for patient-derived cell collection. Advances in gene editing and cell engineering are also making these therapies more scalable, allowing more patients to benefit from them. As research progresses, CAR T-cell therapy is poised to become a broader and more accessible treatment option across oncology.
5. Next-Gen Immunotherapy: Unlocking New Cancer Treatments
Immunotherapy has transformed cancer care, but many patients still do not respond to existing treatments. Research is now advancing new immune checkpoint inhibitors like LAG-3, TIGIT, and TIM-3 to overcome resistance and improve outcomes. The combination of anti-LAG-3 and anti-PD-1 has already extended survival in melanoma patients.
Beyond checkpoints, mRNA vaccines and nano-delivery systems are making immunotherapy more precise and effective. Tumor microenvironment (TME) modulators are also gaining traction, helping dismantle cancer’s immune defenses and enhance treatment response.
New technologies like bispecific T-cell engagers (BiTEs) and trispecific antibodies are revolutionizing how T cells target tumors. Companies like Merck & Co. are investing in CD19 bispecifics, signaling rapid innovation in the field. With these advancements, immunotherapy is evolving into a more personalized and powerful weapon against cancer.
Conclusion: Pioneering the Future of Oncology Clinical Trials
As we move into a new era of cancer research, oncology clinical trials are driving transformative breakthroughs. The innovations explored in this article—ranging from antibody-drug conjugates to AI-driven drug discovery and decentralized trial models—are reshaping the way we approach cancer care. These breakthroughs not only accelerate the development of novel therapies but also enhance patient access, improve treatment precision, and streamline clinical trial efficiency.
At Cromos Pharma, we remain dedicated to supporting groundbreaking research that brings life-changing treatments to patients. As oncology trials evolve, we are committed to collaborating with our sponsors to harness these innovations and push the boundaries of what’s possible in cancer research. The future of oncology is being written today, and together, we can play a pivotal role in shaping it.
To explore how Cromos Pharma can support your oncology clinical trial, contact us at inquiry@cromospharma.com. Let’s work together to advance cancer research and improve patient outcomes worldwide.






























