
World Heart Day 2022
Today is World Heart Day, which strives to increase awareness of cardiovascular diseases. This observance was established in 1999 by the World Health Organization (WHO) and the World Heart Federation (WHF); more than 90 countries participate in this event annually. Currently, this day is the world’s largest campaign to raise awareness about cardiovascular health.
It may come as a surprise to many that cardiovascular diseases (CVDs) are the leading cause of death worldwide, resulting in an estimated 17.9 million deaths each year. This group includes the disorders of heart and blood vessels. 85% of all CVDs are due to strokes and heart attacks, so it is imperative to know the difference between the two:
- Heart Attack – Damage to the heart muscle caused by a loss of blood supply due to blocks in the arteries.
- Stroke – Occurs when the supply of blood to the brain is reduced or blocked completely, which prevents brain tissue from getting oxygen and nutrients.
Symptoms
It is very important to know the different symptoms peculiar to a heart attack and stroke in order to administer people first aid in time.
Common symptoms of a heart attack:
- pain in the center of the chest;
- pain in the arms, the left shoulder, elbows, jaw, or back;
- breathing difficulty;
- nausea or vomiting;
- light-headedness;
- a cold sweat;
- turning pale.
The most important symptoms of stroke include:
- sudden weakness of the face, arm, or leg
- difficulty speaking or understanding speech
- difficulty seeing;
- difficulty walking, loss of coordination;
- severe headache;
- fainting or unconsciousness.
Individuals suffering from any of the symptoms listed above should seek immediate medical attention.
Risk Factors
Overall, more than 4 out of 5 deaths from heart diseases are associated with modifiable risk factors, which can be reduced with lifestyle changes or medical care. There are different risk factors which increase the probability of cardiovascular diseases including:
- High systolic blood pressure (hypertension)
- High LDL cholesterol (raised cholesterol)
- High fasting plasma glucose (diabetes)
- Kidney dysfunction (renal failure)
- Tobacco use
- Unhealthy diet
- Obesity
- Physical inactivity
- Alcohol abuse
Cardiovascular Clinical Trials
There are now 12,800 ongoing clinical trials in the cardiovascular field (clinicaltrials.gov). The most common CVDs in clinical trials are strokes (2031), heart failure (1316), and myocardial ischemia (1161). All of these conditions affect the structure and function of the heart and blood vessels.
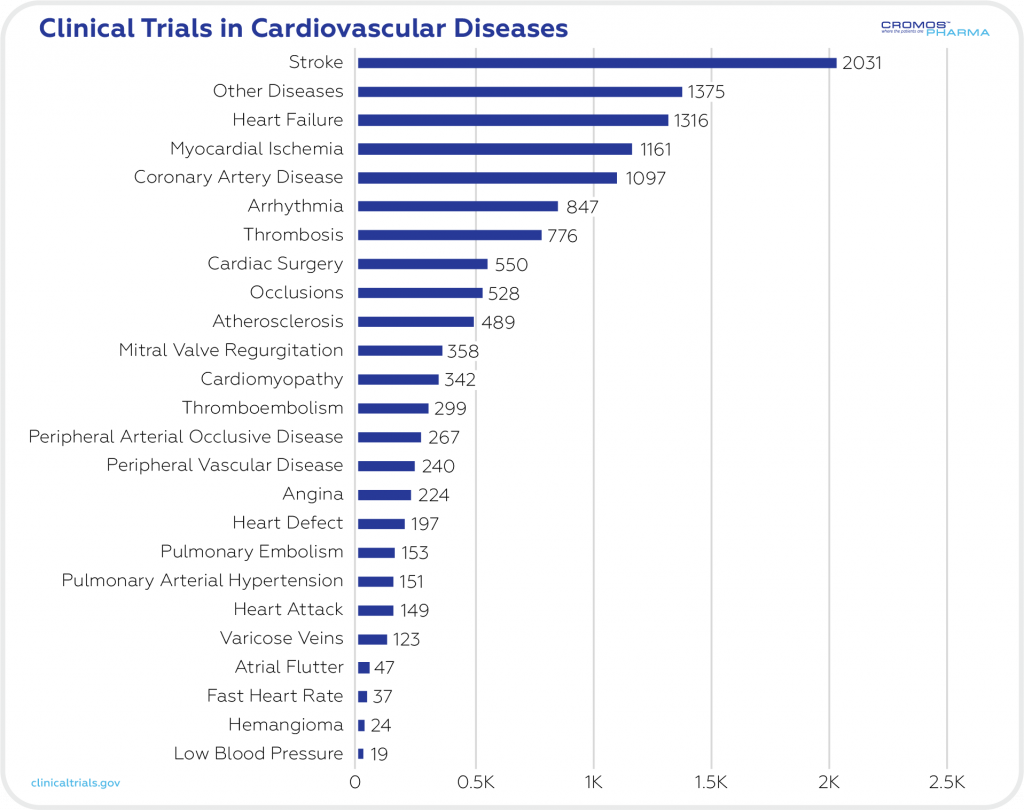
Statistics
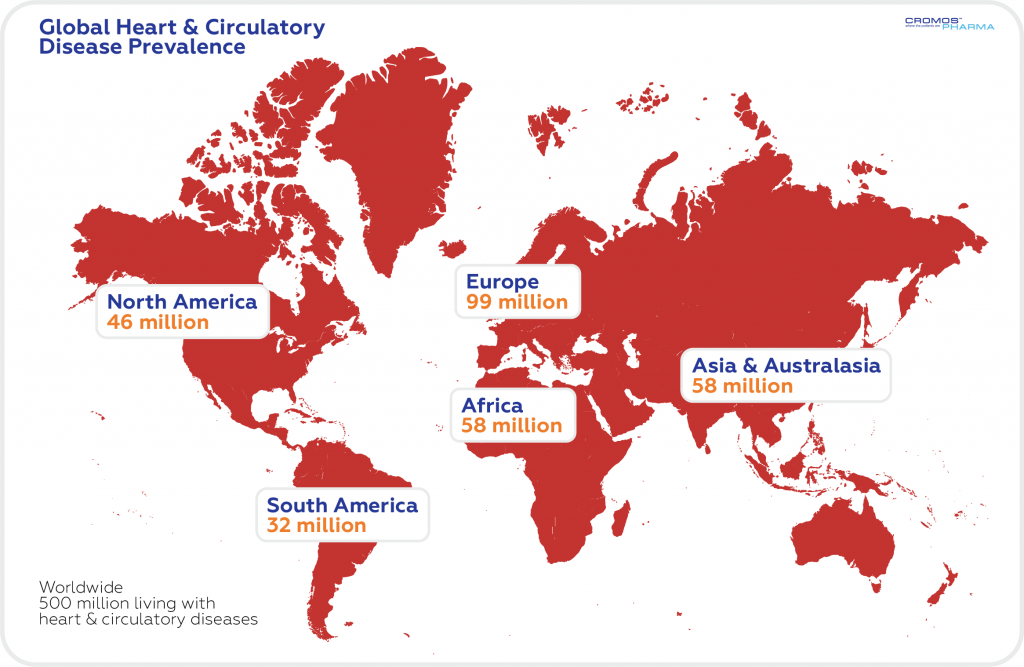
According to the statistics, around 550 million people live with heart and circulatory diseases in the world (1 in 14 people). Cardiovascular diseases are most commonly found in the people of Asia & Australasia (310 million). Since 1990, there has been an estimated 93% increase in the number of people with heart and circulatory disease worldwide.
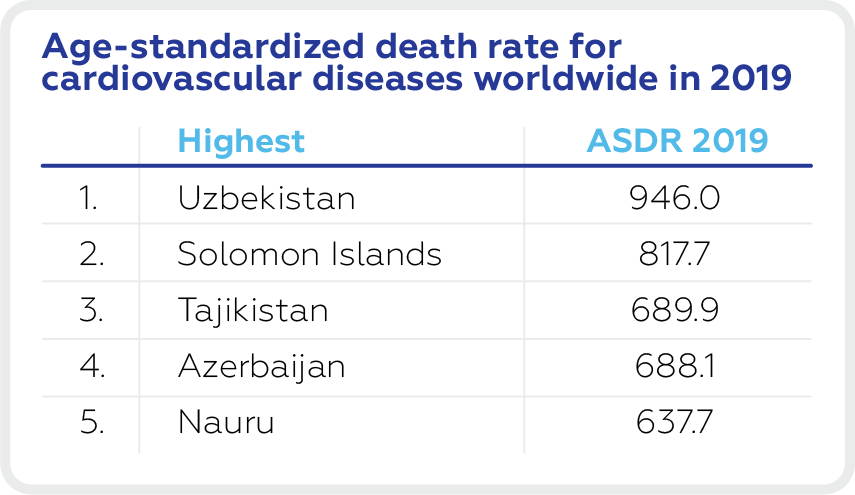
The highest age-standardized death rate for cardiovascular diseases worldwide in 2019 is in Uzbekistan (946), The Solomon Islands (817.7), Tajikistan (689.9), Azerbaijan (688.1) and Nauru (637.7).
Cromos Pharma has extensive experience in managing all aspects of clinical trials in cardiovascular diseases in all phases, I, II, II, and post-market/observational studies. Cardiovascular studies represent nearly 15% of all our work.
About Cromos Pharma
Cromos Pharma is a US-based, international contract research organization delivering fully integrated clinical research solutions, in all trial phases, across a wide range of therapeutic indications. Our expert team, comprised of 95% MDs, has extensive expertise in study design, medical writing, regulatory affairs, site management, patient recruitment and data management.
Cromos Pharma has experience in delivering success in a wide range of trial types, from biosimilars and generics, to successfully managing trials of novel therapeutics in a wide range of clinical indications. Our team provides full-service solutions to international pharma and biotech companies in high-recruiting regions, assuring exceptional data quality. Cromos Pharma combines global expertise with in-depth experience and knowledge in the US, Central and Eastern Europe, Central Asia, Republic of Georgia, and Türkiye to offer exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of conducted trials.
We provide accelerated study start-up timelines in our regions of operation. Regulatory inspections by FDA and EMA and site audits attest to the highest quality of our clinical data.
Established in 2004, Cromos Pharma has strong regional experience that is supported by a global network of offices. Its international HQ is located in Portland, Oregon, USA and its European HQ is in Dublin, Ireland.






























