
World Mental Health Day: How Clinical Trials are Transforming Mental Health Treatments for the Future
As we mark Mental Health Awareness Day, it is an opportune moment to reflect on how increased investments in clinical trials are fostering innovation in the treatment of mental health disorders. Globally, over 1 billion people are affected by mental health disorders, making mental illness one of the leading causes of disability worldwide, according to the World Health Organization (WHO). In the US alone, millions of people struggle with conditions like depression, anxiety, and bipolar disorder. Each year, over 22.5 million people experience depression, and anxiety disorders affect the lives of 42.5 million adults.
This article will explore the projected market growth and key trends shaping the mental health clinical trials landscape, highlighting how these advancements are set to revolutionize mental health treatments in the coming decade.
Market Size and Growth Trends
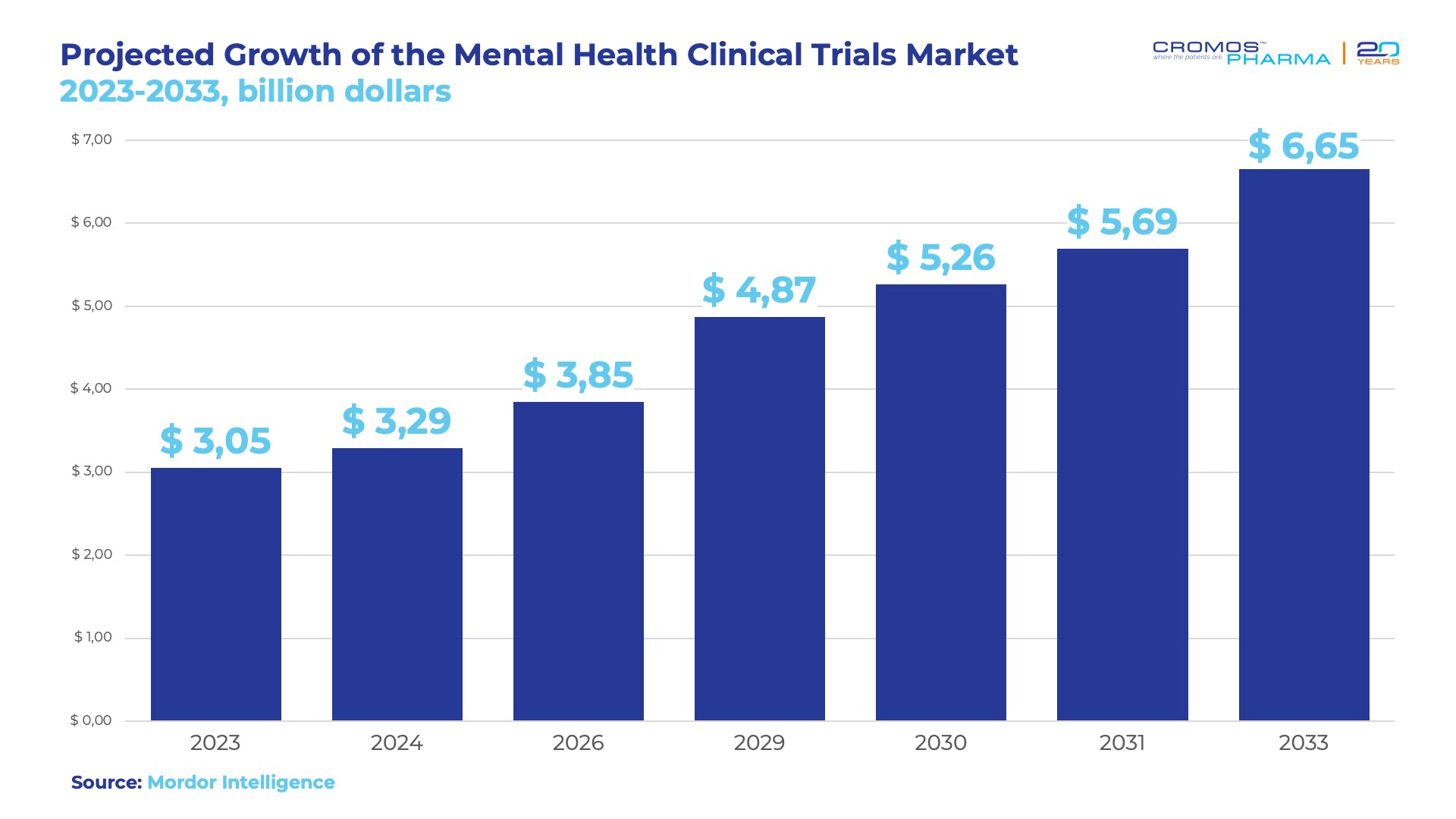
In 2023, the Clinical Trial market was valued at $3.05 billion, and it is projected to grow at a CAGR of 8.11%, reaching approximately $6.65 billion by 2033.
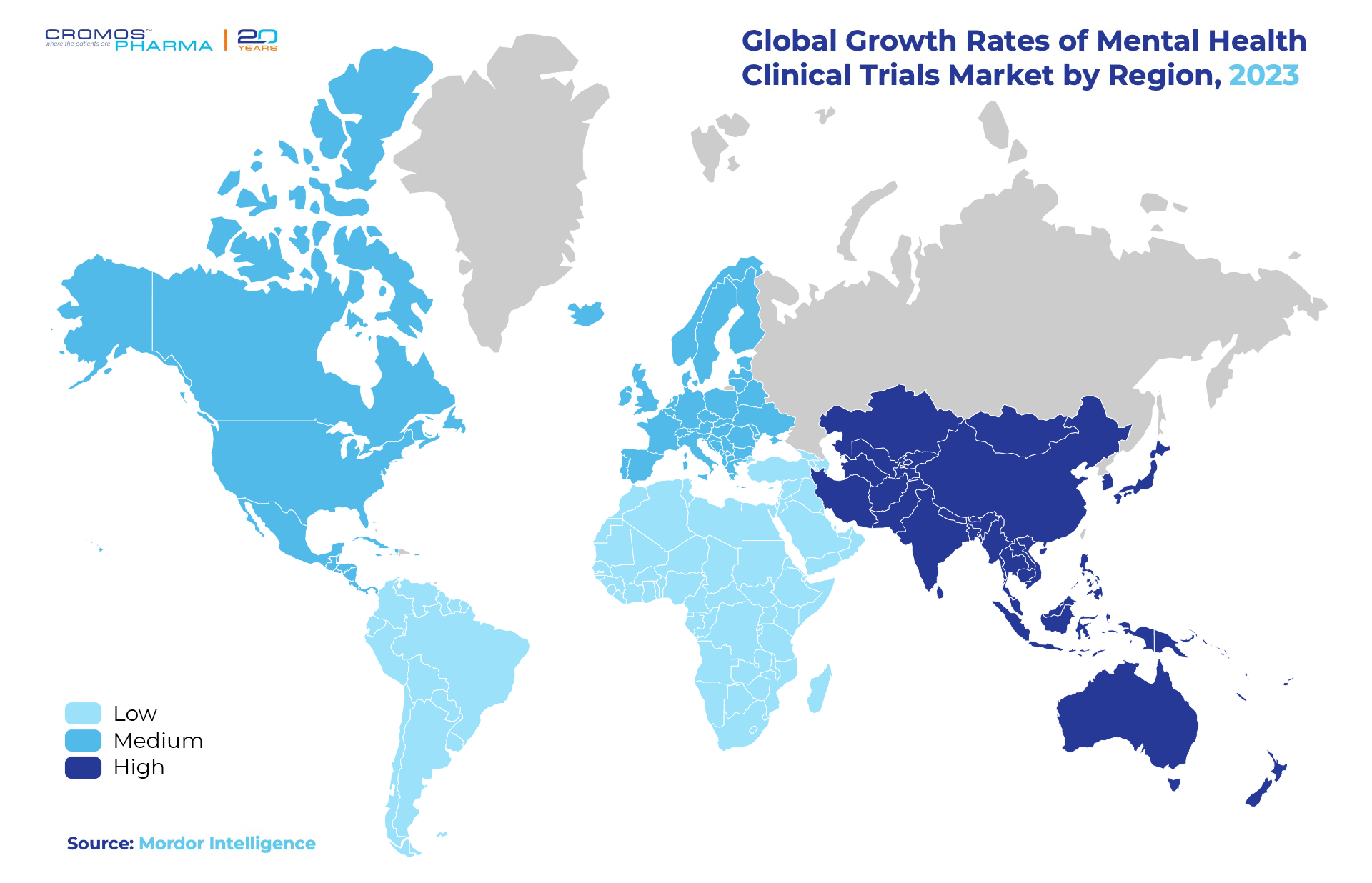
North America remains a leader in the mental health clinical trials market, driven by its robust healthcare infrastructure and clear regulatory framework. Europe follows closely, reflecting its strong clinical research capabilities and well-established mental health services. Meanwhile, Asia Pacific is gaining momentum, expected to grow at the fastest pace with a CAGR of 8.82% during the forecast period, supported by increased healthcare investment and a growing patient population.
Key factors driving the growth of the mental health clinical trials market include:
- Workplace stress increasing the demand for mental health treatments
- Growing awareness reducing the stigma around mental health and encouraging more to seek treatment
- Impact of COVID-19, which intensified mental health issues, pushing investment in clinical research
- Technological advancements like AI and machine learning (ML) improving trial efficiency, patient recruitment, and personalization
US Mental Health Clinical Trials
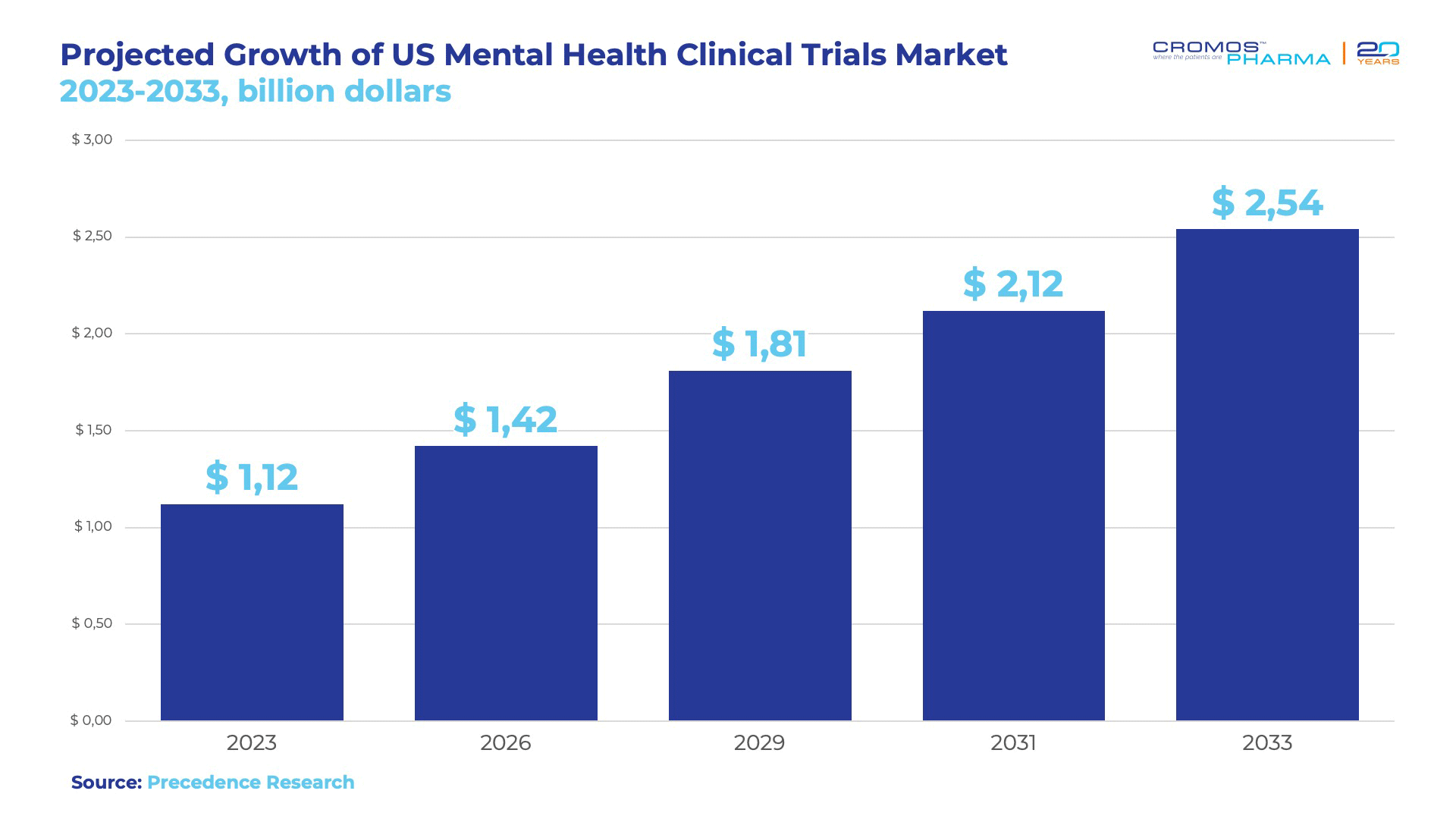
The US market for mental health clinical trials is expanding rapidly, valued at USD 1.12 billion in 2023 and projected to grow to USD 2.54 billion by 2033, with a steady CAGR of 8.53% over the next decade. US dominance in the global market is largely driven by its advanced healthcare infrastructure, which supports cutting-edge clinical research. The US boasts a well-established network of research facilities, academic institutions, and specialized mental health centers, enabling high-quality trials that meet global standards.
Key to the US market’s success is the structured and transparent regulatory framework provided by agencies such as the FDA, which ensures the uniformity and clarity of clinical trial protocols. This encourages both sponsors and researchers to pursue trials in the US, supported by substantial funding from pharmaceutical corporations, biotech companies, academic institutions, and government bodies.
Pharma Giants Leading Innovation in Mental Health Clinical Trials with Strategic Investments
Pharmaceutical companies have long been pivotal in the mental health clinical trials market due to their substantial investments in research and development. These organizations prioritize discovering innovative treatments for conditions like depression, anxiety, schizophrenia, and neurodegenerative disorders. Leveraging cutting-edge research facilities, expert scientific teams, and a strong understanding of regulatory frameworks, they conduct comprehensive clinical trials, pushing new treatments through rigorous testing and approval processes.
As of September 2024, ClinicalTrials.Gov reported that approximately 360 studies on anxiety disorders were being sponsored by biotechnology companies in North America, with an additional 121 studies in Europe. Key industry players such as AstraZeneca, Novartis AG, Pfizer, and Eli Lilly are at the forefront of these initiatives, aiming to develop new treatments for mental health conditions. These efforts are expected to significantly drive the adoption of novel therapies in the coming years, as companies continue investing in clinical trials and research to meet the growing demand for mental health solutions.
The presence of major pharmaceutical companies, including Eli Lilly, Johnson & Johnson, and Pfizer, is pivotal in fueling market growth. These companies are actively engaging in strategic initiatives such as partnerships, mergers and acquisitions, collaborations, and geographic expansions, all aimed at enhancing their services and maintaining a competitive advantage. For instance, in April 2023, CHEPLAPHARM acquired Zyprexa, an antipsychotic medication from Eli Lilly, which is prescribed for conditions like schizophrenia and bipolar disorder.
Moreover, companies are increasingly focusing on strengthening their development pipelines through strategic acquisitions. A notable example is Otsuka Pharmaceuticals’ acquisition of Mindset Pharma in September 2023, which will bolster Otsuka’s research into treatments for anxiety and depression. This trend of expanding portfolios through acquisitions highlights the growing focus on mental health treatments within the pharmaceutical industry.
Key Trends in Mental Health Clinical Trials
As the mental health clinical trials landscape continues to evolve, several key trends are shaping the future of research and treatment development. Below are some of the major trends driving advancements in mental health clinical trials.
- AI’s Growing Role in Mental Health Clinical Trials
AI is transforming mental health clinical trials by improving diagnostics and personalizing treatment approaches. Using ML, AI can process large datasets such as genetic profiles and health records, identifying patterns linked to mental health disorders like anxiety, depression, and schizophrenia. AI tools offer early and accurate predictions, leading to more targeted interventions. For example, Fortis Healthcare launched an AI-powered app in 2024 to support individuals with personalized mental health care solutions.
- Virtual Reality and Gamification in Mental Health Trials
Virtual reality (VR) is rapidly becoming a key tool in mental health clinical trials, offering immersive environments for treatments like exposure therapy. One trend is the gamification of these trials, enhancing patient engagement through interactive, game-like features. A notable example of VR application in clinical research is a study conducted in China that used VR to assess ADHD symptoms in school-aged children. By integrating interactive and immersive elements, the VR-based test provided a more engaging way to evaluate attention deficits and impulsivity, enhancing both accuracy and participant enjoyment.
- Transcranial Magnetic Stimulation (TMS) on the Rise
TMS is emerging as a promising non-invasive therapy for depression and other mental health conditions. Utilizing magnetic fields to stimulate nerve cells in the brain, TMS has demonstrated significant effectiveness, particularly for individuals who have not found relief through traditional treatments. With ongoing research and clinical trials, TMS is poised to become a more widely accepted and accessible treatment option for addressing treatment-resistant mental health disorders. As an example, Neuronetics, Inc. has been utilizing TMS in its FDA-cleared NeuroStar® Advanced Therapy system to treat major depressive disorder (MDD) in patients unresponsive to antidepressants.
In conclusion, the mental health clinical trials market is on a strong growth trajectory, fueled by increasing awareness, advancements in technology, and a growing demand for innovative treatments. With North America leading the market, and Asia-Pacific rapidly catching up, the future of mental health research looks promising. The integration of AI, TMS and VR, alongside major investments from pharmaceutical companies, is set to transform how mental health disorders are treated, making care more personalized and accessible globally.
Cromos Pharma’s Expertise in Mental Health Clinical Trials
Cromos Pharma has a long-standing and deep-rooted experience in conducting clinical trials in the mental health sector. Our expertise in managing complex mental health studies has enabled us to support global sponsors in advancing innovative treatments for a variety of psychiatric conditions, including depression, anxiety, and schizophrenia.
With a focus on maintaining high data quality and rapid patient recruitment, Cromos Pharma’s dedicated team has achieved a remarkable 95% success rate in meeting or exceeding recruitment timelines. Our commitment to excellence and precision has made us a trusted partner for pharmaceutical companies aiming to bring novel mental health treatments to market.
If you have any questions or would like to explore how Cromos Pharma can support your upcoming clinical program, please feel free to reach out via email at inquiry@cromospharma.com. We are committed to advancing your research with precision and expertise.






























