Exploring Clinical Trial Opportunities: Hungary’s 2024 Country Profile
Hungary is recognized for its advanced regulatory framework and state-of-the-art healthcare infrastructure, attracting pharmaceutical companies and clinical research organizations (CROs) to carry out their clinical trials there.
The country’s clinical trial approval process is efficient and streamlined, in line with European Union standards, which substantially shortens the time needed to initiate new trials. As a result, Hungary is currently hosting 1049 clinical trials. The regulation of these trials is under the jurisdiction of the National Center for Public Health and Pharmacy.
Country Overview
Hungary, located in Central Europe, is a landlocked nation spanning 93,000 square kilometers within the Carpathian Basin. It shares its borders with Slovakia and Austria to the north, Ukraine and Romania to the east, Slovenia to the west, and Croatia and Serbia to the south. Budapest, the capital, stands out not only for its population size but also as the hub for the nation’s scientific, academic, and cultural institutions. Other significant cities include Debrecen, Miskolc, and Szeged.
Having been a member of the European Union since 2004, the Hungarians are proud of their ancestry, traditions, culture, and speaking their national Hungarian language.
Demographics
Hungary’s population stands at over 10 million, with a significant portion aged between 25 and 54, representing 42% of the populace. A majority, 72%, live in urban areas, and the median age is 42 years. The primary ethnic groups are the Magyars, followed by Germans, Slovaks, and Romanians, with Magyars making up over 80% of the population. The average life expectancy is 77.3 years.
Healthcare Sector
Hungary has a tax-funded universal healthcare system, organized by the state-owned National Health Insurance fund (NEAK). Except for a small fee, NEAK covers all basic healthcare services, including hospital care, prescription medications, and consultations with general practitioners and specialists.
Hungary is one of the largest pharmaceutical markets in Central and Eastern Europe and the sector accounts for 6.7 % of the national GDP. In recent years the healthcare system has undergone significant changes which resulted in more funding, enhanced working conditions for healthcare professionals, and the introduction of new technologies.
As a result, the country has a well-developed medical research infrastructure with a network of clinical research centers and hospitals to serve the diverse patient population. Hungary had a large pool of experienced investigators who specialize across many therapeutic areas.
Reasons to Conduct Clinical Trials in Hungary
The advantages of selecting Hungary as the destination for conducting clinical research include:
- Hungary’s centralized healthcare system is integral to its ability to rapidly recruit patients.
- A large pool of highly skilled and motivated medical professionals.
- Excellent potential to recruit across a diverse range of therapeutic indications, especially for multi-country trials.
- A proven track record (confirmed by the FDA and EMA audits) of delivering high quality data.
Snapshot of Hungary’s Clinical Trials
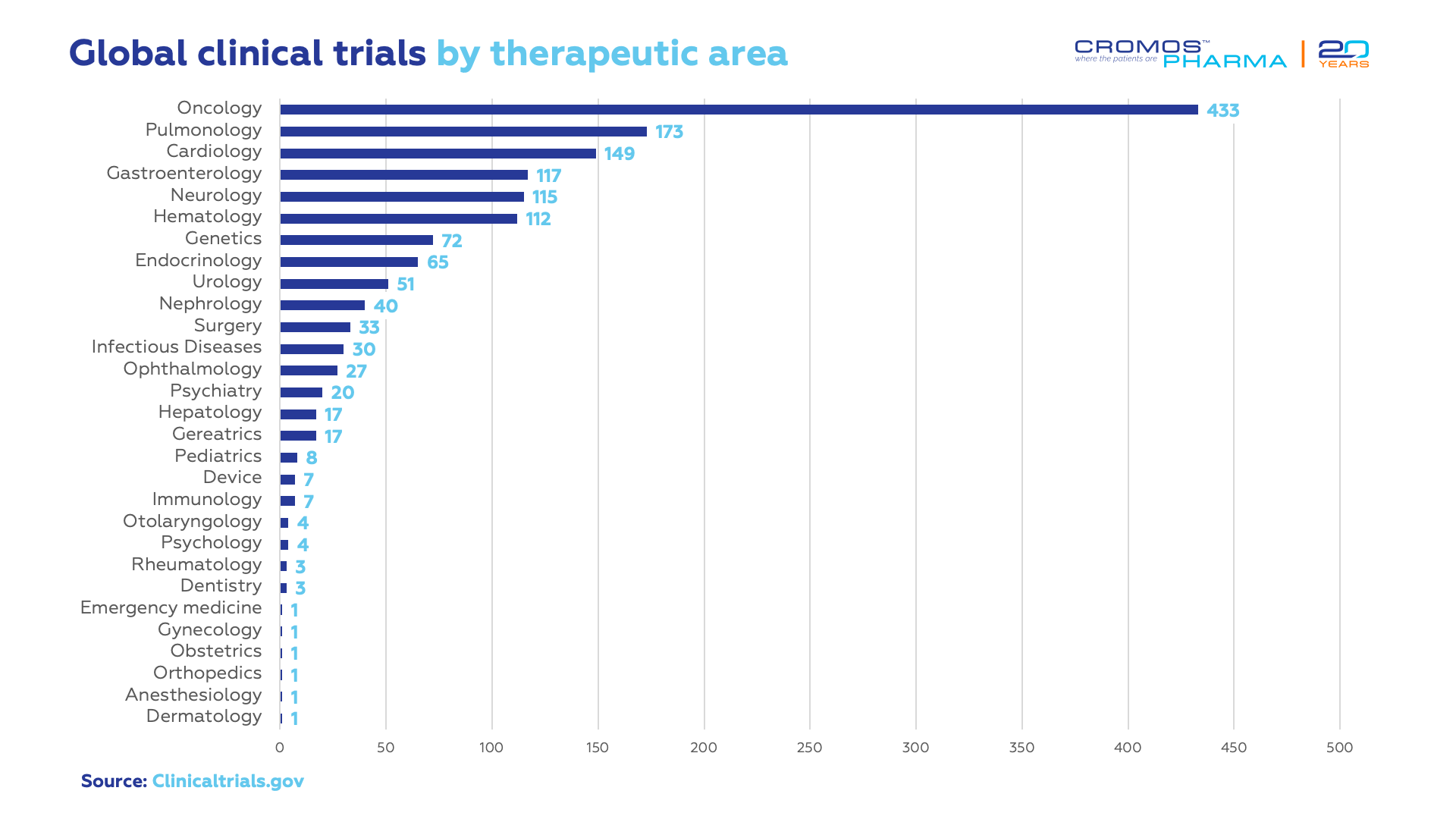
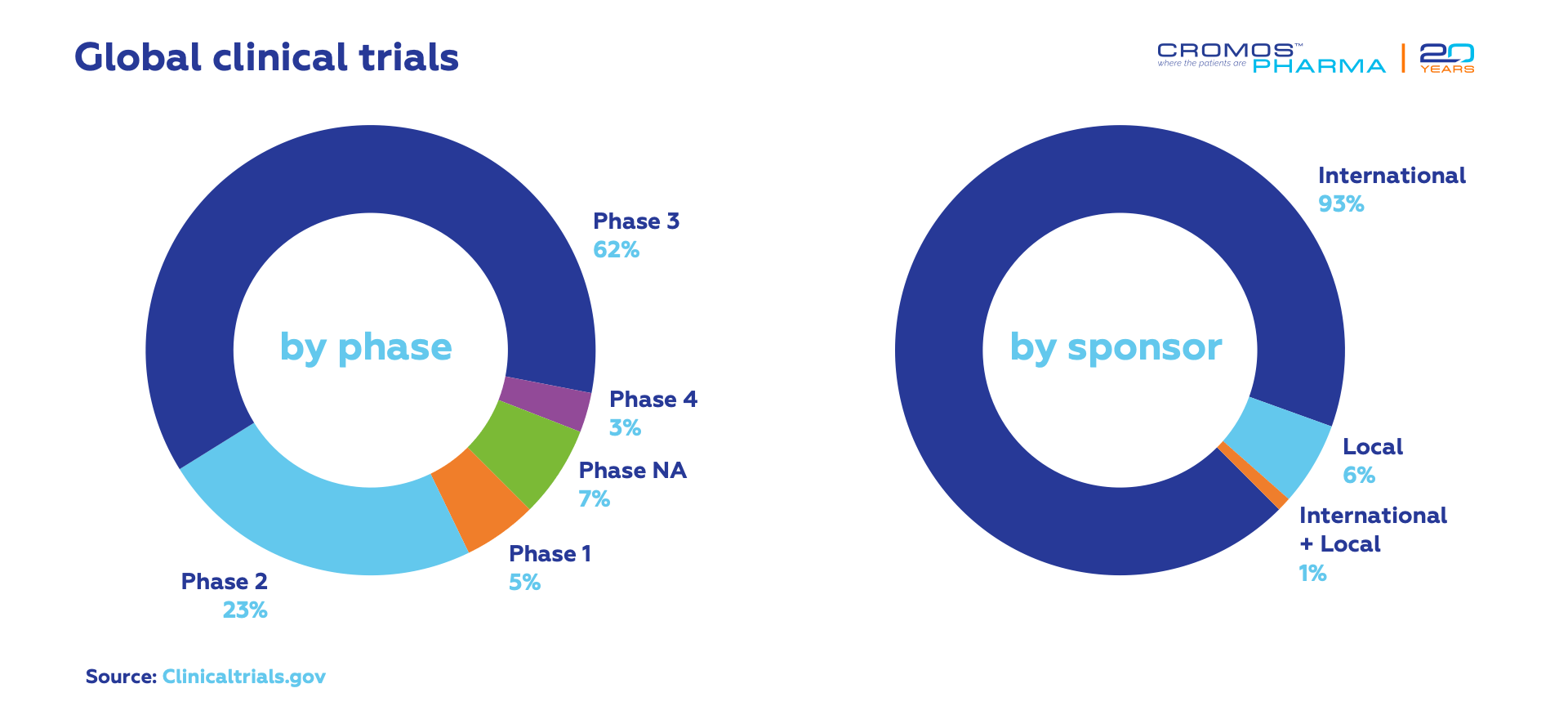
According to data obtained recently from clinicaltrials.gov, the largest number of ongoing clinical trials are conducted in oncology (433), followed by pulmonology (173), cardiology (149), gastroenterology (117) and neurology (115). Most trials are in Phase 3 (629). International sponsors conduct 93% of all ongoing clinical trials.
Hungary: Quick Facts
Regulatory Approval Process
For clinical trials in Hungary, applications must be submitted via the EMA’s CTIS portal and typically undergo a 60-day review. This process includes a parallel assessment of Parts I and II of the application documents within 45 days.
When Hungary acts as the Reporting Member State, its NNGYK plays a crucial role in overseeing the trial’s evaluation, particularly during the initial phases and when formulating conclusions for Part I of the application.
Agreements with Sites and Investigators
Many institutions have adopted a single-contract approach between the sponsor and the trial site, ensuring that investigators and other staff are compensated via their agreements with the institution. While these contracts aren’t required to be submitted to the Ethics Committee or Competent Authority, the estimated budget must be included in the initial Clinical Trial Application through the CTIS portal.
EC Review and Approval
The Hungarian Central Ethics Committee (ETT-KFEB) is the only ethical body involved in the review process of clinical trials via CTIS.
Trial Sites’ Location
Hungary’s highway system is highly developed, allowing for travel to any location within two and a half hours from Budapest. The capital hosts the largest medical facilities, with notable centers also in university cities like Debrecen, Pécs, and Szeged. The National Institute of Oncology, situated in Hungary, is distinguished internationally for its cancer research and treatment excellence.
Legal Entity
Non-EU sponsors must appoint an EU legal representative for conducting trials in Hungary. While having a CRO representative isn’t compulsory for applications in Hungary, certain sections of the CTIS application form must be completed in Hungarian. Patient-related documents should also be submitted in either Hungarian or in both English and Hungarian. Given these requirements, the support of local staff is essential for ensuring compliance and facilitating the application process.
QP Declaration / GMP Certificate
For non-EU manufactured Investigational Products the Declaration from the IMP importer’s Qualified Person (QP) stating that the manufacturing site operates in compliance with the EU GMP is required for submission.
Documents Requiring Special Attention
The Informed Consent Form (ICF) must be tailored to include country-specific details and translated into Hungarian. Additionally, special regulations regarding genetic testing, biobanking, patient insurance, and compensation/reimbursement must be adhered to.
The Hungarian Central Ethics Committee has set forth specific requirements for the patient card, impacting the Patient Informed Consent process.
Investigators must possess a GCP certificate that is no more than 5 years old. Moreover, they must submit a Protocol Agreement Form (PAF) affirming their understanding of the investigational plan and their commitment to compliance.
When listing study sites, applicants must provide the exact Hungarian names and specify the Organization ID and Local ID identifiers generated in EMA’s Organization Management Service (OMS). Additionally, completing a site suitability form is obligatory for trial submissions.
Official Language
Essential documentation should be submitted in English. Patient-related documents, labels and the protocol synopsis must be translated into the Hungarian language.
Patient Insurance
Clinical trial insurance, which covers health injuries related to the trial participants, is mandatory and must be included as part of the CTIS Application. The insurance certificate serves as proof of coverage. Additionally, it is necessary to have a local contact person from the insurer company available for participants to communicate within the Hungarian language.
Useful Tips
Initiating budgeting and contracting should occur early in the preparation phase. Contracts may be executed prior to obtaining regulatory authorization but will only be deemed valid upon study approval. Typically, the contracting process takes longer than regulatory or EC authorization.
Although offering a fee to patients for their participation (stipend) is restricted for phase I and PK (sub)trials, patient reimbursement is generally required for most clinical trials.
Regulatory Environment for Clinical Trials in Hungary
A registry of clinical trials conducted in Hungary is available on the NNGYK website, providing information on trials authorized by the Hungarian Medicines Authority. This registry simplifies the search for trials that have obtained necessary permissions or are already underway.
Further clinical trial-related details can be obtained from the “EU Clinical Trials Register” and the CTIS public interface. We recommend using the EudraCT number to ensure consistency between the registry and the register.
Hungary, as a member of the EU/EEA, adheres to EU Clinical Trials Regulation no 536/2014. Clinical Trial Applications must be submitted through the EMA’s CTIS portal, and communication with the Competent Authority and the Central Ethics Committee occurs via the CTIS interface. Part I and Part II documents can be submitted concurrently or within two years of Part I authorization. The assessment of these documents typically takes place in parallel within 45 calendar days, with a possible extension of up to 76 calendar days for inquiries requiring further clarification.
Cromos Pharma – Your Partner in International Clinical Research
Cromos Pharma boasts a proficient local team adept at effectively overseeing both regulatory and contracting matters, ensuring swift initiation of studies. We enlist highly skilled and knowledgeable personnel, guaranteeing that each trial managed in Hungary yields excellent data quality and dependable results.
The company seamlessly melds global proficiency with comprehensive local insight, resulting in exceptional patient recruitment. Our team has either met or shortened enrollment timelines in 95% of the trials conducted.
If you are contemplating conducting clinical trials in Hungary or any other country where Cromos Pharma operates, our team is eager to address any queries you may have.