
Clinical Trials in Hungary – Country Profile for 2023
Hungary is known for its well-developed regulatory environment and advanced healthcare system. This has made the country a popular destination for pharmaceutical companies and contract research organizations (CROs) to conduct their clinical trials.
Aligned with the European Union regulations, the approval process for clinical trials is streamlined and efficient, significantly reducing the timelines for starting new trials. Consequently, there are currently 1044 clinical trials being conducted in the country. All clinical trials in Hungary are regulated by the National Institute of Pharmacy and Nutrition (OGYÉI). If Hungary is selected/designated as Reporting Member State (RMS), OGYÉI is responsible for approving clinical trial applications via Clinical Trials Information System (CTIS) and ensuring that all trials are conducted in accordance with GCP guidelines.
Country Overview
Hungary is a landlocked country in central Europe. Spanning 93 000 square kilometers of the Carpathian Basin, it is bordered by Slovakia and Austria to the north, Ukraine and Romania to the east, Slovenia to the west, and Croatia and Serbia to the south. Hungary’s capital is the city of Budapest. The capital dominates the country both by the size of its population and by the concentration of most of the its scientific, scholarly, and artistic institutions. Other noteworthy cities are Debrecen, Miskolc and Szeged.
Having been a member of the European Union since 2004, the Hungarians are proud of their ancestry, traditions, culture, and speaking their national Hungarian language.
Demographics
The current population of Hungary is 9.5 million with 42% of the population between the ages of 25 and 54. 72% of the residents reside in urban areas. The major ethnic groups in Hungary are the Magyars, Germans, Slovaks, and Romanians. The Magyars are the dominant group, outnumbering the others by over 80%. The average life expectancy is 77.3 years.
Healthcare sector
Hungary has a tax-funded universal healthcare system, organized by the state-owned National Health Insurance fund (NEAK). Except for a small fee, NEAK covers all basic healthcare services, including hospital care, prescription medications, and consultations with general practitioners and specialists.
Hungary is one of the largest pharmaceutical markets in Central and Eastern Europe and the sector accounts for 7.5% of the national GDP. In recent years the healthcare system has undergone significant changes which resulted in more funding, enhanced working conditions for healthcare professionals, and the introduction of new technologies.
As a result, the country has a well-developed medical research infrastructure with a network of clinical research centers and hospitals to serve the diverse patient population. Hungary had a large pool of experienced investigators who specialize across many therapeutic areas.
Reasons to Conduct Clinical Trials in Hungary
The advantages of selecting Hungary as the destination for conducting clinical research include:
- Hungary’s centralized healthcare system is integral to its ability to rapidly recruit patients.
- A large pool of highly skilled and motivated medical professionals.
- Excellent potential to recruit across a diverse range of therapeutic indications, especially for multi-country trials.
- A proven track record (confirmed by the FDA and EMA audits) of delivering high quality data.
Snapshot of Hungary’s Clinical Trials
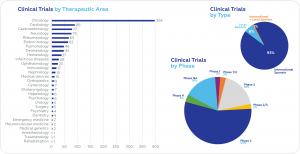
According to data obtained recently from clinicaltrials.gov, the largest number of ongoing clinical trials are conducted in oncology (395), followed by cardiology (88), gastroenterology (77) and neurology (75). Most trials are in Phase 3 (575). International sponsors conduct 93% of all ongoing clinical trials.
Hungary: Quick Facts
Regulatory approval process the approval process starts when the submission
Applications must be uploaded into the EMA’s CTIS . The review and approval of applications require 60 days. To achieve this, Parts I and II of the documents are assessed in parallel within 45 calendar days.
Agreements with sites and investigators
Two agreements require a signature: one with an institution and the other with a PI. Contracts do not have to be submitted to EC/CA, however the estimated budget per patient is required.
EC review and approval
CEC (ETT-KFEB) is the only ethical body involved in the review process via CTIS.
Trial sites’ location
Hungary has a well-developed highway infrastructure and all sites can be reached by car within two and a half hours. The largest medical centers are in Budapest, with many reputable centers located in university towns such as Debrecen, Pécs, and Szeged. The National Institute of Oncology is the most renowned cancer center in Hungary and has high international acclaim.
Legal entity
For non-EU sponsors an EU legal representative is required. It is not mandatory to have a CRO representative as the applicant for Hungary. However, CTIS application form contains sections that should be filled out in Hungarian language. Submissions to CEC also need to be done in either Hungarian or in both English and Hungarian. The assistance of local staff is therefore recommended.
QP Declaration / GMP certificate
Declaration from the QP stating that the manufacturing site operates in compliance with the EU GMP is required for submission.
Documents requiring special attention
Informed Consent Form (ICF) in the Hungarian language should be customized with country-specific information.
Investigator’s GCP certificate should not be older than 5 years.
The investigator’s statement on knowledge of the investigational plan and intention to comply (Protocol Agreement Form, PAF) should be attached.
List of study sites displayed with the exact Hungarian name, where the applicant also indicates the Organization ID and Local ID identifiers generated in OMS.
Site suitability form is mandatory.
Official language
Essential documentation should be submitted in English. Patient-related documents, labels and the protocol synopsis must be translated into the Hungarian language.
Patient insurance
Local insurance certificate should be submitted. It is a requirement that a local contact (for the patient to contact in Hungarian language) should be available.
Useful tips
Budgeting and contracting should commence early in the preparation phase. The contracts can be signed before regulatory authorization is obtained, and will only be deemed valid when the study gets approved. The contracting process usually takes longer than regulatory/EC authorization. Patient reimbursement is a requirement that applies to most clinical trials.
Regulatory environment for Clinical Trials in Hungary
Registry of clinical trials taking place in Hungary: The list of clinical trials authorized in Hungary can be found on the NIPN website. It contains data on clinical trials approved by the Hungarian Medicines Authority that are running at the country’s research sites. The list simplifies the search for clinical trials that have received the required permission or have already been initiated.
The “EU Clinical Trials Register” and the CTIS public interface can be used to obtain the important clinical trial-related details. We recommend using the EudraCT number of the test identifiers to ensure consistency between the list and the register.
Hungary is an EU/EEA member, and it follows EU Clinical Trials Regulation no 536/2014. The Clinical Trial Application must be submitted through the EMA’s CTIS. Communication takes place via the CTIS interface. It is possible to submit Part I and Part II documents at the same time, or Part II within two years of authorization. Part I and II documents are assessed in parallel within 45 calendar days and up to 76 calendar days if there are any questions requiring an extension.
Cromos Pharma – your partner in International Clinical Research
Cromos Pharma has an experienced local team that effectively manages regulatory and contracting processes to ensure that studies can be initiated in the shortest period possible. We recruit highly educated and experienced staff that assures that each trial managed by our team in Hungary produces exceptional data quality and reliable results.
Cromos Pharma combines global expertise with in-depth local experience and knowledge that translates into exceptional patient recruitment. Our team has met or reduced enrollment timelines in 95% of the conducted trials.
If you are considering conducting clinical trials in Hungary or in any of the other countries where Cromos Pharma is present, our team will be happy to answer your questions.






























