
Conducting Clinical Trials in Poland – Country Profile 2021
Poland is the largest clinical trials market in central and Eastern Europe. Cromos Pharma has been managing clinical research in Poland since 2015 and opened a permanent office in Warsaw in February 2020.
WHY POLAND?
- Strong track record over 20 years of producing high quality data.
- Large population (38.5 million) in comparison with neighboring countries offers great potential for patient recruitment.
- Large proportion of treatment-naïve patients in a wide range of therapeutic areas.
- Patients eager to participate in clinical trials as a means of accessing novel therapies.
- Highly skilled, qualified, experienced and motivated investigators and site staff.
- Large network of specialized medical facilities located around major urban centers.
- EU member state since 2004.
- Lower costs – on average 30% less than US – due in part to efficiencies in patient recruitment and comparatively lower salaries and fees.
BACKGROUND
- Poland is a country located in Central Europe with a population of 38.26 million.
- Poland is the sixth most populous member state of the European Union.
- Its largest city and capital is Warsaw.
- It is a high-income and developed economy and the sixth largest in the EU. In 2020, its GDP dipped to $594.2 billion, the first fall in almost two decades, due to the COVID-19 pandemic.
- It is, however, one of the EU economies least affected by the pandemic and the World Bank predicts a return to growth in 2021.
- Poland has a well-diversified economy with a strong clinical research sector and attracts significant numbers of international sponsors to conduct trials there.
- Overall, Poland is perceived as a good place to carry out clinical research due to several advantages including a large population, and significant naïve patient populations in a diverse range of clinical areas.
- Its membership of the EU, advanced economy, and high standard of medical care add to positive perceptions about conducting trials there. It also has a strong track record across several decades of producing high quality data corroborated by regulatory authorities including FDA and EMA.
SKILLED CLINICAL PROFESSIONALS AND STRONG HEALTHCARE INFRASTRUCTURE
A well-educated clinical workforce with experience in ICH GCP-compliant clinical trials allows for efficient recruitment of skilled investigators. Most public hospitals are well disposed towards taking part in trials and have established protocols for working with international sponsors. Poland also boasts excellent access to sophisticated diagnostic tools and laboratory evaluations often required in the conduct of global trials.
POSITIVE PATIENT ATTITUDES TO TRIALS
In general, Polish investigators and their patients are favorably inclined to participate in trials as a way of accessing novel therapies not yet available through their national health system.
GOVERNMENTAL SUPPORT FOR THE SECTOR
Polish authorities are actively seeking to grow the country’s clinical research sector e.g. to make site contracting, regulatory approvals and other processes more streamlined, and to encourage patients to take part in global trials.
A SNAPSHOT OF CLINICAL TRIALS IN POLAND JANUARY 2020 – JANUARY 2021
Using clinicaltrials.gov our team analyzed the trends in the clinical trials market in Poland between 1 January 2020 and 1 January 2021.
A total of 372 trials were initiated between January 2020 and January 2021 in comparison with 392 clinical trials the previous year. This slight decline may be attributed to disruption caused by the COVID-19 pandemic.
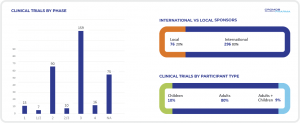
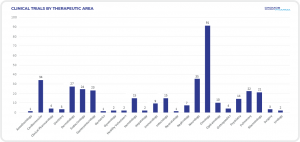
GENERAL INFORMATION ABOUT CLINICAL TRIAL APPROVAL PROCESS IN POLAND
Regulatory Agency/Competent Authority (RA) and Ethics Committee (EC) approval is required prior to any new drug or non-CE marked medical device clinical trial initiation in Poland. The Clinical Trial Application (CTA) should be submitted to the RA by the clinical trial sponsor or its authorized representative.
Application for drug studies can be made to RA and EC in parallel, however, the CTA for medical devices must be made to the RA after EC approval has been obtained.
RA requires that the contracts of all participating sites in Poland must be signed and submitted to RA before the CTA submission for medical devices research or between the CTA submission and the end of 60-day period of CTA approval process for human drugs research.
From all Principal Investigators participating in the study in Poland a Country Coordinator must be chosen and his/her local EC becomes the Central EC (CEC) responsible for review and approval of the proposed study.
If the local EC gives favorable reviews on the proposed sites or doesn’t respond within 2 weeks, then the study is considered approved for all sites submitted within the application.
STREAMLINING OF REGULATORY PROCESSES
In 2019, in response to a stagnation in the number of clinical trials carried out in the country, Polish authorities announced changes to the regulatory processes for clinical trials aimed at improving efficiencies in study approval and start-up times.
A new agency, the Medical Research Agency (ABM), was established in 2019, to streamline clinical trial processes and promote clinical research in Poland.
The agency is a specialized body of experts working to promote innovation in Polish medicine, focusing on areas related to oncology, hematology and rare diseases.
The activities of the agency primarily consist of co-financing scientific research and development work as well as interdisciplinary projects, with emphasis on clinical, observational and epidemiological research.
These actions, alongside simplification of the European Union regulatory procedures for clinical trials, have improved the outlook for the Polish clinical trials sector.
CONCLUSION
With high levels of patient recruitment, an established framework for conducting clinical trials, a large population of skilled clinical professionals and reputation for producing high quality data, Poland remains a key location for international sponsors. Recent moves to establish a more streamlined approach to regulatory approval and initiatives to promote patient participation in clinical research have further strengthened Poland’s position in the clinical trials sector. Cromos Pharma opened its permanent office in Poland February 2020 to further develop and expand its operations in this country.
ABOUT CROMOS PHARMA
- Cromos Pharma provides tailored and effective clinical trial services to support the development of drugs that transform healthcare.
- Established in 2004, it is an international CRO offering fully integrated services with expertise in delivering all aspects of clinical trials in all clinical phases and a range of therapeutic areas.
- Cromos Pharma delivers rapid recruitment and excellent patient retention as well as expert study design and management.
- Cromos Pharma has strong regional experience in Central and Eastern Europe. Its international HQ is in Portland, Oregon and EU base in Dublin, Ireland.
If you would like to find out more about how Cromos Pharma support your clinical trials in Poland, email: bd@cromospharma.com.
Read our pdf here.






























