
Сlinical trials in the Czech Republic – Country Profile 2021
Cromos Pharma began working in the Czecя Republic (Czechia) in the 2010s and established a representative office in Prague in 2015.
The country has a strong track record in clinical research delivering high quality data. In recent years, it has moved towards becoming a location for more niche clinical trials including those focused on cell and gene therapies and rare diseases.
BACKGROUND
- The Czech Republic is a high-income country with a population of 10.6 million and GDP of $250.69 billion (World Bank, 20191).
- The country has a strong pharmaceutical sector and established clinical research infrastructure.
- Its largest city and capital is Prague with a population of 1.3 million.
- Czechia boasts a high density of potential clinical trial sites with over 250 public hospitals, a significant network of public outpatient clinics and a growing number of specialized private health clinics.
NATIONAL HEALTH PROFILE
- Life expectancy at birth 79.1 years
- Ischemic heart disease and stroke are the leading causes of death in the Czech Republic.
- Lung cancer is the leading cause of death among cancers in Czechia with smoking remaining a major public health issue.
- Other leading causes of death include Alzheimer’s Disease, COPD and pancreatic and breast cancers.
CZECHIA AS A LOCATION FOR CLINICAL TRIALS ADVANTAGES
Czechia boasts several significant advantages for international sponsors considering the country as a location for clinical research including:
- Solid clinical research infrastructure
- High density of clinical sites
- High standards in education (particularly clinical
and medical training) - Supportive regulatory framework
- Well-organized centralized healthcare system
- Large pool of skilled clinical professionals trained in GCP that produces high quality data
- Significant treatment-naïve patient populations in a diverse range of therapeutic areas.
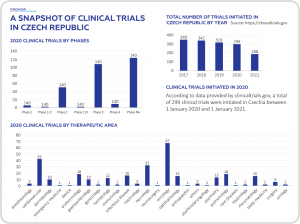
CLINICAL TRIAL REGULATORY AND APPROVAL PROCESS
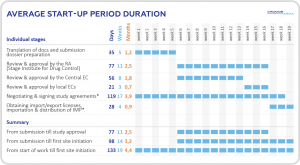
Czechia’s regulatory authority for clinical trials is the State Institute for Drug Control (SUKL). It has a reputation for being open, efficient and supportive in its approach to supporting clinical research. This allows for quick approval periods and expedited start-up timelines (on average 60 days).
IMPACT OF EUCTR ON REGULATION
 The way clinical trials are conducted in the EU will undergo a major overhaul when the Clinical Trial Regulation (Regulation (EU) No 536/2014) comes into effect. The Regulation harmonizes the assessment and supervision processes for clinical trials throughout the EU via a publicly available Clinical Trials Information System (CTIS; formerly the EU Clinical Trial Portal and Database). The European Medicines Agency (EMA) will set up and maintain the new information system, in collaboration with the member states and the European Commission. It is currently estimated that the Regulation will enter into effect by January 2022.
The way clinical trials are conducted in the EU will undergo a major overhaul when the Clinical Trial Regulation (Regulation (EU) No 536/2014) comes into effect. The Regulation harmonizes the assessment and supervision processes for clinical trials throughout the EU via a publicly available Clinical Trials Information System (CTIS; formerly the EU Clinical Trial Portal and Database). The European Medicines Agency (EMA) will set up and maintain the new information system, in collaboration with the member states and the European Commission. It is currently estimated that the Regulation will enter into effect by January 2022.
CONTACT US
Cromos Pharma has a highly experienced team based in Prague who effectively manage all regulatory and contracting processes to ensure that studies can get up and running in the quickest time possible. Our team also has an impressive track record for rapid patient recruitment and retention as well as proven relationships with a skilled pool of investigators and staff who consistently produce the highest quality data.
ABOUT CROMOS PHARMA
Cromos Pharma provides tailored and effective clinical trial services to support the development of drugs that transform healthcare. It is an international CRO with over 16 years’ experience in offering fully integrated services and delivering all aspects of clinical trials in all clinical phases and a range of therapeutic areas. Cromos Pharma delivers rapid recruitment and excellent patient retention as well as expert study design and management. Cromos Pharma has strong regional experience in Central and Eastern Europe. Its US base is in Portland, Oregon with additional operations in South Florida and its European HQ is situated in Dublin, Ireland.
For more information about running your clinical trial in the Czech Republic please email bd@cromospharma.com to set up an introductory call.
Read our pdf here.






























