Cromos Pharma and Avance Clinical share details of innovative collaboration at BIO-Europe 2020
Avance Clinical and Cromos Pharma, with operations in Australia and Central/Eastern Europe, respectively, are successfully collaborating on a scalable clinical solution for biotechs.
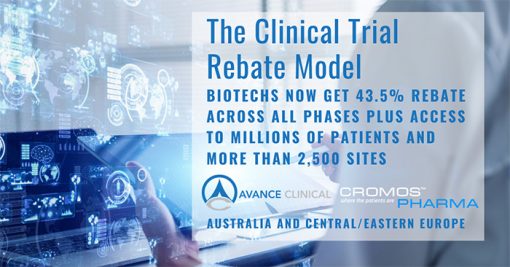
The collaboration allows biotechs to quickly start their pre-IND early phase studies in Australia, then expand to Central/Eastern Europe to access the large patient populations for their Phase 2 and 3 studies. Importantly, the AU/EU model allows biotechs to keep their data and trial management in Australia and take advantage of the Australian cash rebate of up to 43.5% on clinical trial costs, throughout all study phases, across both regions.
“We are very excited about this model that seamlessly incorporates two of the most compelling and complementary international clinical research hubs for our biotech clients from early to late phase studies,” said Yvonne Lungershausen, CEO of Avance Clinical. “Our eClinical and early phase expertise is well augmented by Cromos Pharma’s track record in late stage trials and rapid enrolment capabilities,” she continued.
“We have been collaborating with Avance Clinical and referring clients to each other for years,” remarked Vlad Bogin, CEO of Cromos Pharma. “But it was a true Eureka moment when we realized that combining Australia’s 43.5% tax rebate with Central/Eastern Europe’s unbeatable recruitment rates is a paradigm shift in how clinical trials should be conducted,” he concluded.
For more information email inquiry@cromospharma.com