
Medical Writing Guidelines
What Is Medical Writing?
The dynamic field of medicine involves extensive research, which needs to be communicated to various stakeholders. One common way to achieve this objective is through medical writing.
In this article, you will be introduced to different facets of medical writing and their specific applications. Furthermore, you will have a preview of medical writing services available for any of your needs.
Overview of Medical Writing
A lot of people mistake medical writing for science journalism. While their subject matter may coincide, the former is highly technical while the latter is intended for lay people.
Medical writing will be discussed here as a specialized genre of science communications that is classified into two subtypes: publication and regulatory writing.
Publication Writing
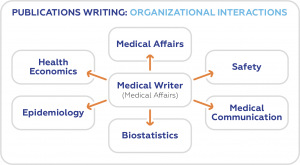
Publications may refer to any scientific articles aimed at communicating research output to a niche audience such as clinicians, scientists, or Pharma and biotechnology investors. Examples of such publications include medical journal articles, publication plans, conference abstracts, and poster presentations.
Those who specialize in publication writing are referred to as medical writers. They come from various professional backgrounds – doctors, life scientists, and bioengineers, among others. Although they have particular specialties, they all write to present real-world evidence about a drug, a medical procedure, or equipment to various stakeholders.
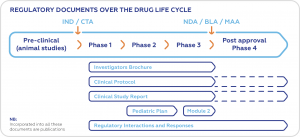
 For instance, the pharmaceutical industry goes through five steps to complete the life cycle of a drug discovery project. Thorough documentation and writing skills are required for each of the phases, from the pre-clinical trials to the post-approval observations.
For instance, the pharmaceutical industry goes through five steps to complete the life cycle of a drug discovery project. Thorough documentation and writing skills are required for each of the phases, from the pre-clinical trials to the post-approval observations.
Medical writers are also required to be adept in other skills parallel to scientific research and medical writing. They should at least be familiar with health economics, biostatistics, epidemiology, pharmacovigilance, and general medical affairs.
Regulatory Writing
A narrower and more closely-monitored subtype of medical writing is regulatory writing. In contrast to scientific publications, regulatory documents have to meet the standards set by the government agencies, such as the Food and Drug Administration and European Medicine Agency. These documents contain a different set of medical terminology and communication style.
Regulatory writers come up with clinical protocols, study reports, common technical document (CTD) modules, investigator’s brochures, and investigation plans. They also prepare clinical trial lay summaries, patient information sheets and informed consent forms, package inserts for patients, and plain language summaries for broad audience, including patients and professionals in adjacent disciplines.
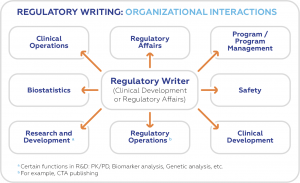 The role played by regulatory writers for pharmaceutical and biotechnology companies cannot be downplayed. Just like publications, regulatory documents are required for the different stages of the drug life cycle clinical trials. Government agencies also scrutinize these documents before they can approve the release of a new drug or medical device to the market.
The role played by regulatory writers for pharmaceutical and biotechnology companies cannot be downplayed. Just like publications, regulatory documents are required for the different stages of the drug life cycle clinical trials. Government agencies also scrutinize these documents before they can approve the release of a new drug or medical device to the market.
Because of the high standards expected of regulatory writers, they continually hone their skills through courses provided by professional organizations (i.e. American Medical Writers Association (AMWA), European Medical Writers Association (EMWA)), regulatory agencies, and continuing professional education programs worldwide.
Guidelines and Principles Governing Publications and Regulatory Documents
Medical writing generally needs to adhere to specific guidelines and principles. Becoming a medical writer is a serious responsibility because it entails a strong sense of ethics and professionalism.
For publications, the core value to emphasize is integrity that is guided by various organizations within the community of medical science. The International Committee of Medical Journal Editors (ICMJE) outlines the recommended criteria for authorship and publishing transparency. The International Society for Medical Publication Professionals (ISMPP) similarly proposed a set of good publication practices (GPP3) guidelines.
Moreover, regulatory medical writing is also subject to the rules and guidelines imposed by the International Council for Harmonisation or ICH. This global body seeks to harmonize the various scientific and technical aspects of drug registration.
The ICH-specified guidelines are divided into four categories: quality, safety, efficacy, and multidisciplinary. Some of the more critical guidelines include E3 (Clinical Study Reports), E6 (Good Clinical Practice), and M4 (Common Technical Document). Many templates are available on the ICH website for the use by regulatory writers.

In Need of a Medical Writer?
Given how rigorous medical writing is, the task of preparing clinical documents for publication or regulatory submission necessitates the expertise of professional medical writers.
Both start-ups and established biotech companies that are engaged in drug discovery research understand the importance of engaging experienced and ethical medical writers.
Seeking Help from the Experts
Even very experienced medical professionals understand that medical writing requires a very different skill set as compared to other aspects of drug discovery and clinical program management. Not all healthcare professionals and researchers have the acuity for medical writing.
Even if you have doctors and scientists in your group, it would still be prudent to have a dedicated medical writer on board. These writers specialise in producing the documents that are relevant to any given stage of your preclinical or clinical development programs.
Medical writers are experts in communicating ideas and transforming data into an appropriate format that is tailored to the intended stakeholders. In addition to their background in the medical field, their analytical and fact-checking skills add tremendous value to the final product that they create.
Start Small
You do not always need to hire an in-house medical writer from the outset. Often, especially if you are a young company, you may opt to source professional medical writing services from accredited providers. This is a cost-efficient and scalable option. You should always seek a medical writing services provider with a relevant background, appropriate skill set and established reputation in the industry.
Cromos Pharma: Your Partner in Medical Writing
Cromos Pharma is a full-service international contract research organization providing medical writing services to clients worldwide. It is driven by its focus on giving biotechnology and pharmaceutical companies solutions to accelerate its drug development while maintaining the highest level of quality.
Our medical writing team is comprised of MD’s and Ph.D.’s in medical sciences. Over the past 18 years they have crafted innumerable number of documents for all phases of clinical trials in a wide range of therapeutic indications.
If you have any questions or want to find out more about how Cromos Pharma can support your next clinical program please get in touch with us by emailing inquiry@cromospharma.com.






























