
The Not so Rare “Rare” Diseases
Current and future challenges in clinical research of rare diseases
What are rare diseases?
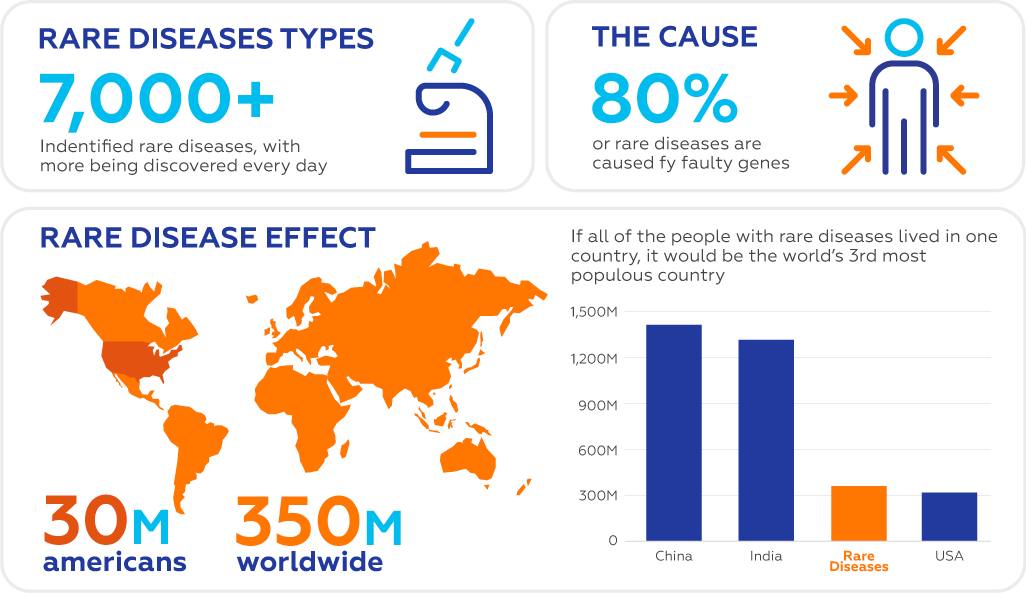
As implied in its name, rare diseases affect small percentages of patients. However, when factoring in the multitude of rare diseases, the overall number of people affected by them is a staggering 475 million worldwide. According to the National Institutes of Health, about 25-30 million Americans have over 6,800 rare diseases, which amounts to 1 in 10 people. Over 50% of individuals with rare disease conditions are children, which means that over 200 million children in the world are currently suffering. It is important to note that over 80% of rare diseases have a genetic disorder as its cause or predisposing factor, which is partially the reason why only about 5% have clinically approved treatments.
In Europe there are over 36 million people living with one of over 6000 rare diseases. It is important to note that the same disease may be considered endemic in one region and rare in another. For instance, thalassemia is caused by a genetic mutation, which is rare in Northern Europe but very prevalent in the Mediterranean region.
In the US a rare disease is defined as a disease or condition that affects fewer than 200,000 people. In the EU, a rare disease is defined as a condition that affects no more than 1 person in 2,000.
Although uncommon as a single entity, cumulatively rare diseases have a major impact on the affected population, a profound influence on their quality of life, and a significant economic burden.
Why rare diseases are an important medical and social issue?
Rare diseases are an important public health issue and a challenge for the medical community. Often called ‘orphan’, rare diseases have been neglected for many years. Eventually, with the help of several interest groups the 1983 Orphan Drug Act in the USA was passed. It put forth a number of initiatives that stimulated the development of orphan drugs. Since its enactment, more than 600 orphan drugs and biological products have been approved just in the US alone. Furthermore, public awareness about the difficulties of patients with rare diseases was first raised by the report of the National Commission on Orphan Disease of the US Government in 1989. The Commission’s findings highlighted the following issues:
- Difficulties in patients’ care
- The lack of information on rare diseases
- Difficulties in financing research
- Lack of adequate health insurance and coverage of medical expenses
- The limited availability of effective treatments.
The use of the collective term, rare diseases, is used to include a very heterogeneous group of disorders, often with genetic mutations which are often severely debilitating, drastically affect life expectancy, and impair both cognitive and physical abilities. This translates into a reduced quality of life, learning disabilities, and low earning capacity. One example of this is inborn errors of metabolism, most of which are rare with a prevalence of 1 in 5000 live births. A prospective long-term study showed that between 1985–97, of 1935 newborn babies with inborn errors of metabolism, only 11% reached adulthood.
Furthermore, rare diseases pose a substantial burden on the affected families. A further study was conducted whereby this burden was evaluated by sending quality-of-life questionnaires to 2500 patient families with chronic diseases of which 8.2% had rare diseases. The findings showed that the relatives of patients with rare disorders had a more pronounced loss of social and economic opportunities and worse experience with the healthcare system.
The challenges of rare diseases
In addition to being a burden to patients and their families, rare diseases are very challenging from the perspective of clinicians, researchers, and biotech/pharma companies.
The challenges facing clinicians who care for impacted individuals include acquiring knowledge and experience in caring for such patients, and the availability of local experts and of expert guidelines. The challenges to researchers are in gathering adequate patient cohorts and garnering funding for their research. Biotech and pharma often see the pursuit of rare disease indications as high-risk propositions given the often binary outcome of the very expensive clinical programs.
In pursuit of these challenges, the dedicated perseverance of patients and clinical/scientific communities to improve care and develop new understanding has had a major impact. Some of the achievements include the formation of powerful patient advocacy bodies which have brokered partnerships between the patient, scientific communities, the government, and pharma/device companies in service of detection, optimal care, and research; procurement of funds to support research; formation of collaborative consortia of clinicians and scientists; and general activation of the respective patient communities to continue to foster these successes.
The future of rare disease research and treatment still requires enhanced detection techniques, dissemination of understanding concerning optimal care, and research to prevent, treat, and cure disease.
Future of Rare Diseases Research
The primary focus of past initiatives was devoted to developing research studies or gathering information on rare diseases. The end goal of these initiatives was to understand the requirements and limitations of conducting clinical trials with small patient populations. Present and future modalities have expanded the role of patient advocacy organizations and patient engagement in all aspects of clinical research. This has further driven acceptance within the research community as a serious public health issue.
Going forward, a better understanding and interpretation of available information from multiple sources including electronic health records and big data sources will be the next primary focus of rare disease research. Novel interventions that combine personalized genetic treatments and advances in clinical trial design and data analyses will pave the way for novel orphan drugs.
Another facet worth mentioning is the expansion of diagnostics with an emphasis on improved genetic sequencing methodologies, which should allow to diagnose rare diseases much earlier.
From a clinical research perspective, improvements in patient recruitment and increased flexibility in approval procedures by regulatory agencies will facilitate faster approvals. Specifically, children will gain the most as early diagnosis and treatment may prevent the development of later complications of their diseases.
The recent advent of Decentralized Clinical trials (DCTs), wearable devices, remote sensors, and the development of mobile device applications will all assist in the continuous monitoring of patients receiving investigational drugs. Telemedicine is already providing critical access to expert clinicians with a better insight into individual rare diseases.
All this translates into a more effective, diverse, and omnichannel research methodology which in turn will benefit patients and their families.
Cromos Pharma and Rare Disease Clinical Trials
At Cromos Pharma, we prioritize the success of rare disease studies for our sponsors. Our robust and trusted systemic approach ensures that our clients achieve their development goals. We comprehensively address and solve the following challenges:
- Understanding all patient experiences to help us better identify and recruit trial participants.
- Utilizing our extensive and comprehensive investigator network to identify the most optimal sites.
- Providing risk-based monitoring and additional support to sites.
- Leveraging effective strategies to retain patients throughout our studies.
- Keen understanding of the regulatory practice and various regulatory requirements in each country of our operations.
In the last 10 years Cromos Pharma has contributed to the design, operational management, data collection, and analysis of 20+ Phase II-IV studies in rare diseases, including but not limited to:
- Autism spectrum disorders
- Bulimia nervosa
- Congenital afibrinogenemia
- Growth hormone deficiency (GHD)
- Glioblastoma
- Hemophilia A
- Hemophilia B
- Hereditary angioedema
- Niemann-Pick disease Type C
- Meniere’s disease
- Retinitis pigmentosa
- Sjogren-Larsson syndrome
- X-linked adrenomyeloneuropathy
- Von Willebrand’s Disease
Our strong and professional team consists of medical and regulatory experts with long-term experience and deep knowledge of this challenging area.
The distinctive methodology and detailed feasibility analysis used for site selection allow us to develop a realistic approach to patient recruitment for each individual study. In their analysis, Cromos Pharma’s Feasibility Team performs comprehensive diligence on patient eligibility in addition to reviewing other key aspects of the project, such as Investigators’ experience, availability of equipment, competing trials, variability of standards of care, and regulatory aspects in particular countries.
Additionally, rare disease drug development requires a lot more attention and resources to educate the sites and the investigators by providing additional training and support throughout the study duration.






























