
World Diabetes Day 2022
World Diabetes Day was established in 1991 by the International Diabetes Federation (IDF) and the World Health Organization (WHO) in response to the growing concerns about the increasing health threat posed by diabetes. It is celebrated each year on the 14th of November, the birthday of the co-discoverer of insulin, Sir Frederick Banting.
World Diabetes Day is intended to increase access to diabetes education and help improve the lives of the more than half a billion people living with this disease worldwide.
Diabetes is a chronic disease, which is characterized by a high level of blood glucose. The hormone insulin allows the blood sugar into the body’s cells to be used for energy. With diabetes, the body doesn’t make enough insulin or can’t use it properly, and excess glucose stays in the bloodstream. There are 3 main types of diabetes: type 1, type 2 and gestational diabetes (pregnancy-related). More than 95% of people with diabetes have type 2 diabetes.
Certain factors increase the risk of type 2 diabetes including family history, being overweight, insufficient physical activity, and poor diet. Lifestyle changes can prevent the development of type 2 diabetes. Unfortunately, preventive measures don’t apply to type 1 diabetes.
Statistics
According to the statistics, 537 million adults are living with diabetes (1/10 of the world population). The global diabetes prevalence is constantly growing. The number of diabetes cases is predicted to rise to 643 million by 2030 and 783 million by 2045.
In 2021, 6.7 million people died of diabetes.
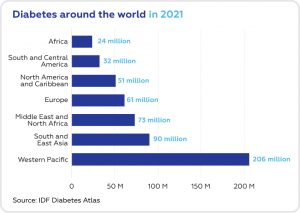
In total, 37.3 million Americans live with diabetes (11.3% of the population), and 96 million adults (1/3 of the population) have prediabetes. Unfortunately, more than 8 in 10 of them don’t realize they have it.
Clinical Trials in Diabetes
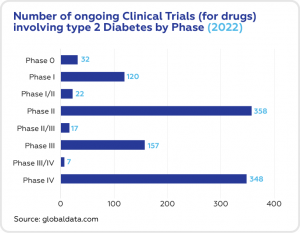
Type 2 diabetes is highly researched. The global pharmaceutical industry is steadily conducting new clinical trials aimed to produce better drugs for this dangerous disease. The largest number of ongoing clinical trials for type 2 diabetes are conducted in the Asia-Pacific region, Europe and North America.
There are currently 1,061 ongoing clinical trials that deal with type 2 diabetes. 358 are in Phase 2, 348 trials are in Phase 4 and 157 are in Phase 3.
Diabetes Management
- careful blood glucose control
- Insulin for people with type 1 diabetes
- oral medications and sometimes insulin for type 2 diabetes
- blood pressure control
- screening for early signs of diabetes-related kidney disease
- screening and treatment of diabetic retinopathy
- management of dyslipidemia
- foot care
Cromos Pharma has extensive experience in managing all aspects of clinical trials in diabetes mellitus, in all phases, including rescue, post-marketing and observational studies. Endocrinology trials represent a substantial part of company’s research portfolio.
About Cromos Pharma
Cromos Pharma is a US-based, international contract research organization delivering fully integrated clinical research solutions, in all trial phases, across a wide range of therapeutic indications. Our expert team, comprised of 95% MDs, has extensive expertise in study design, medical writing, regulatory affairs, site management, patient recruitment and data management.
Cromos Pharma has experience in delivering success in a wide range of trial types, from biosimilars and generics, to successfully managing trials of novel therapeutics in a wide range of clinical indications. Our team provides full-service solutions to international pharma and biotech companies in high-recruiting regions, assuring exceptional data quality. Cromos Pharma combines global expertise with in-depth experience and knowledge in the US, Central and Eastern Europe, Central Asia, Republic of Georgia, and Türkiye resulting in rapid patient recruitment. Our team has met or reduced enrollment timelines in 95% of conducted trials.
We provide accelerated study start-up timelines in our regions of operation. Regulatory inspections by FDA and EMA and site audits attest to the highest quality of our clinical data.
Established in 2004, Cromos Pharma has strong regional experience that is supported by a global network of offices. Its international HQ is in Portland, Oregon, USA and its European HQ is in Dublin, Ireland.






























